What determines the salinity of sea water
Seeing a little higher figure 3.5 ppm
, you might think that this is a constant for any sea water on our planet. But everything is not so simple, salinity depends on the region. It just so happened that the further north the region is located, the greater this value.
The south, on the contrary, boasts not so salty seas and oceans. Of course, all rules have their exceptions. Salt levels in the seas are usually slightly lower than in the oceans.
What can be the reason for geographic division? It is not known, researchers take it for granted, there is everything. Perhaps the answer should be sought in the earlier periods of the development of our planet. Not at the time when life was born - much earlier.
We already know that the salinity of water depends on the presence of:
- magnesium chloride.
- sodium chloride.
- other salts.
Perhaps, in some parts of the earth's crust, the deposits of these substances were somewhat larger than in neighboring regions. On the other hand, no one canceled the sea currents, sooner or later the general level had to level off.
So, most likely, a small difference is associated with the climatic features of our planet. Not the most unfounded opinion, if you remember the frosts and consider what exactly water with a high salt content freezes more slowly.
Freezing stages
It is very interesting to watch how sea water freezes. It is not immediately covered with a uniform ice crust, like fresh water. When part of it turns into ice (and it is fresh), the rest of the volume becomes even more salty, and an even stronger frost is required to freeze it.
Ice types
As the sea cools, different types of ice form:
- snowstorm;
- sludge;
- needles;
- Salo;
- nilas.
If the sea has not yet frozen, but is very close to it, and at that time snow falls, it does not melt when it comes into contact with the surface, but is saturated with water and forms a viscous mushy mass called snow. Freezing, this porridge turns into sludge, which is very dangerous for ships caught in a storm. Because of it, the deck is instantly covered with an ice crust.
When the thermometer reaches the mark necessary for freezing, ice needles begin to form in the sea - crystals in the form of very thin hexagonal prisms. Collecting them with a net, washing off the salt and melting them, you will find that they are insipid.
When it gets even colder, the fat begins to freeze and forms an ice crust, as transparent and fragile as glass. Such ice is called nilas, or bottle. It is salty, although it is formed from unleavened needles. The fact is that during freezing, the needles capture the smallest drops of the surrounding salt water.
Only in the seas is there such a phenomenon as floating ice. It arises because the water here cools faster off the coast. The ice formed there freezes to the coastal edge, which is why it was called fast ice. As frost intensifies during calm weather, it quickly captures new territories, sometimes reaching tens of kilometers in width. But as soon as a strong wind rises, the fast ice begins to break into pieces of various sizes. These ice floes, often huge (ice fields), are carried by the wind and current throughout the sea, causing problems for ships.
Desalination of sea water.
Regarding desalination, everyone has heard at least a little, some now even remember the film “Water World”. How realistic is it to put one such portable distiller in every house and forever forget about the problem of drinking water for humanity? Still fantasy, not reality.
It's all about the energy expended, because for efficient operation huge capacities are needed, no less than a nuclear reactor. A desalination plant in Kazakhstan operates on this principle.The idea was also submitted in the Crimea, but the power of the Sevastopol reactor was not enough for such volumes.
Half a century ago, before numerous nuclear catastrophes, it was still possible to assume that a peaceful atom would enter every home. There was even a slogan. But it is already clear that no use of nuclear micro-reactors:
- In household appliances.
- At industrial enterprises.
- In the construction of cars and aircraft.
- And yes, within the city limits.
Not expected in the next century. Science may take another leap and surprise us, but so far these are just the fantasies and hopes of careless romantics.
Freezing point of distilled water
Does distilled water freeze? Recall that for water to freeze, it is necessary to have some crystallization centers in it, which can be air bubbles, suspended particles, as well as damage to the walls of the container in which it is located.
Distilled water, completely devoid of any impurities, does not have crystallization nuclei, and therefore its freezing begins at very low temperatures. The initial freezing point of distilled water is -42 degrees. Scientists managed to achieve supercooling of distilled water to -70 degrees.
Water that has been exposed to very low temperatures but has not crystallized is called "supercooled". You can place a bottle of distilled water in the freezer, achieve hypothermia, and then demonstrate a very effective trick - see the video:
By gently tapping on a bottle taken from the refrigerator, or by throwing a small piece of ice into it, you can show how it instantly turns into ice, which looks like elongated crystals.
Distilled water: does this purified substance freeze or not under pressure? Such a process is possible only in specially created laboratory conditions.
ÑаÑÑолÑемпеÑаÑÑÑа
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · α α ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð δ¶¶Ð Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð δ °ÑÑвоÑа ÑвелиÑиÑÑÑ. Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ññμ¼ññññññ ° ° ° ° ° °ñññ °ñññ °ññ °ñññ Ð ÐμÐ Ð Ð Ð Ð Ð Ðμ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ »Ð ²Ðððð ооо²²²²²² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ²Ð Ð Ð ² ÐÑÐ »Ð¸ ÑоÐ'ÐμÑжР° ниÐμ Ñол и в ÑÐ ° ÑÑоР»Ðμ мÐμнÑÑÐμ, ÑÐμм ÑÑÐμÐ ± ÑÐμмоÐμ Ð'л Ñ ÑвÑÐμкÑиÑÐμÑкого ÑÐ ° ÑÑвоÑÐ °, воÐ'Ð ° ÑÑкоÑÐ¸Ñ Ð¾Ð ±Ñазование кÑиÑÑаллов лÑда пÑи оÑлажденÑÑаѸлда пÑи оÑлажденÑÑаиоиии Running ñðμμðððμð½ððÐμÐμРРо Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ¸ÑÐ¸Ð²Ð°ÐµÑ ÐºÐ¾Ð½ÑенÑÑаÑÐ¸Ñ Ñоии оÑÑалÑном ÑÑÑаÑÐ¸Ñ Ñои оÑÑалÑном ÑÑÑаÑÐ¸Ñ Ñои оÑÑалÑном ÑÑÑаÑÐ¸Ñ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ñ ññ ñ ñ Ð ° Ð ° ñ °
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ñÐ ññ ñ ñ ñ ññ ° ñ ° Ð ° С Ð Ð'Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð μ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð μñ ÐÐ ° мÐμÑÐ · Ð ° ниÐμ ÑвÑÐμкÑиÑÐμÑкого ÑÐ ° ÑÑвоÑÐ ° â ÑÑо пÑоÑÐμÑÑ Ñ ÑÑÐ ° ÑÑиÐμм ÑкÑÑÑой ÑÐμпР»Ð¾ÑÑ, поÑÑÐ¾Ð¼Ñ ÑÐμмпÐμÑÐ ° ÑÑÑÐ ° оÑÑÐ ° ÐμÑÑÑ Ð½ÐμиР· менной. Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ Ð ñ °Ð Ð Ð Ð Ðμ ñ ñ ² Ð ñ Ð Ð Ð ² Resume. Ð Ð · ñ ñ ñ ñ ñ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ññÐ Ð ° Ð ° Ð Ð Ð Ð'Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° ¸ поÑÑоÑннойÐÐ ° ннÑй пÑоÑÐμÑÑ Ð¿ÑимÐμнÑÐμÑÑÑ Ð² пР»Ð ° ÑÑинÑÐ ° ÑÑÑ Ð¸ÑпР° ÑиÑÐμл ÑÑ Ð¿Ñи ÑÑÐ ° нÑпоÑÑиÑовкÐμ пÑоÐ'ÑкÑов .
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð Ð ° Ð ° Ð Ð Ð Ð ° Ð ° Ð ° калÑÑÐ¸Ñ Ð¸ ÑлоÑида наÑÑиÑ. Ð Ð ° ÑÑÐ²Ð¾Ñ ÑÐ »Ð¾ÑиÐ'Ð ° кР° л ÑÑÐ¸Ñ Ð¿ÑÐμжÐ'Ðμ вÑÐμго иÑпоР»ÑÐ · ÑÐμÑÑÑ Ð² пÑомÑÑл ÐμннÑÑ Ð¿ÑоÑÐμÑÑÐ ° Ñ Ð¿ÑÐ ¸ замоÑаживании пÑодÑкÑов, ÑÑанении и Ñ.д. 17.8 °С. Ð ° Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð 55 °C Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · Ð (μ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ¿Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñññððμμ'''ðððððððð °Ð²Ð°ÑÑÑоÑÑкий пÑодÑкÑам Ðð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÑÑÑонÑÐ°ÐºÑ ÑаÑÑооа ÑоÑлаждаемÑм пÑодÑкÑом.
Ð Ð ° ÑÑÐ²Ð¾Ñ ÑÐ »Ð¾ÑиÐ'Ð ° нР° ÑÑÐ¸Ñ (пиÑÐμвой Ñол и) нÐμ поÑÑÐ¸Ñ Ð¿Ð¸ÑÐμвÑÐμ пÑоÐ'ÑкÑÑ. оñððð Ð ° Ð °ñÐ Ð Ð Ð ðñððРРРРРРРРРРРРРРРРРРРРРРРРРРРРоÐμ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð Ð Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ Ð ° Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² РРРРизиÑелÑно в21.1 °С. Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ·
THE WATER CYCLE IN THE WORLD OCEAN
In the polar regions, the water, cooling down, becomes denser and sinks to the bottom. From there, it slowly slides towards the equator. Therefore, at all latitudes, deep waters are cold. Even at the equator, the bottom waters have a temperature of only 1-2 ° above zero.
Since currents carry warm water from the equator to temperate latitudes, cold water rises very slowly from the depths to take its place. On the surface, it warms up again, goes to the subpolar zones, where it cools down, sinks to the bottom and again moves along the bottom to the equator.
Thus, in the oceans there is a kind of water cycle: on the surface, water moves from the equator to the subpolar zones and along the bottom of the oceans - from the subpolar zones to the equator. This process of water mixing, along with other phenomena mentioned above, creates the unity of the oceans.
If you find an error, please highlight a piece of text and click Ctrl+Enter
.
In the section on the question of what can be achieved the lowest temperature of a water-salt solution of ordinary (table, NaCl) salt, given by the author European
the best answer is By adding salt to water, the rate of ice melting increases and the melting temperature of ice falls lower. This is because the addition of salt causes a weakening of the molecular cohesion and the destruction of the crystal lattices of ice. The melting of the ice-salt mixture proceeds with the removal of heat from the environment, as a result of which the surrounding air cools and its temperature decreases. With an increase in the salt content in the ice-salt mixture, its melting point decreases. The salt solution with the lowest melting point is called the eutectic, and its melting temperature is called the cryohydrate point. The cryohydrate point for an ice-salt mixture with table salt is -21.2°C, at a salt concentration in the solution of 23.1% relative to the total mass of the mixture, which is approximately equal to 30 kg of salt per 100 kg of ice.With a further increase in salt concentration, it is not a decrease in the melting temperature of the ice-salt mixture, but an increase in the melting temperature (at a 25% salt concentration in the solution to the total mass, the melting temperature rises to -8 ° C). When freezing an aqueous solution of table salt in a concentration corresponding to cryohydrate point, a homogeneous mixture of ice and salt crystals is obtained, which is called a eutectic solid solution. The melting point of a eutectic solid solution of sodium chloride is -21.2 ° C, and the heat of fusion is 236 kJ / kg. The eutectic solution is used for zero-torque cooling. To do this, a eutectic solution of table salt is poured into zeros - tightly sealed forms - and they are frozen. Frozen zeros are used to cool counters, cabinets, refrigerated portable cooler bags, etc. (open the freezer of a household refrigerator - you will find such a container). In trade, ice-salt cooling was widely used before the mass production of equipment with machine cooling.
Answer from dry out
the lowest temperature of any temperature is absolute zero, around -273 degrees Celsius
Answer from Olya
the temperature depends on the salt concentration in the solution, the higher the concentration, the lower the freezing point. the reference book was taken away from me for a while)) but if we proceed from the fact that sea water is a saline solution, then we can conclude that the freezing temperature is much lower than zero .... degrees -15-20
Answer from able
22.4% NaCl aqueous solution freezes at 21.2 °C
to the question Aqueous solution of NaCl "crystallization temperature"
Answer from Yergey Neznamov
Table 10.8. Freezing point of NaCl solutionNaCl content, g in 100 g of water 5 - -4.4 9.0 - -5.4 10.6 - -6.4 12.3 - -7.5 14.0 - -8.6 15.7 - -9.8 17.5 - -11.0 19.3 - - 12.2 21.2 - -13.6 23.1 - - 15.1 25.0 - - 16.0 26.9 - -18.2 29.0 - -20 .0 30.1 — -21.2
Freezing point of salt water
Experiments with ice for children are always interesting. While conducting experiments with Vlad, I even made several discoveries for myself.
Today we will find answers to the following questions:
- How does water behave when frozen?
- What happens if you freeze salt water?
- coat will warm the ice?
- and some others…
freezing water

Does salt water freeze?
The more salty the water, the lower the freezing point. For the experiment, we took two glasses - in one fresh water (marked with the letter B), in the other very salty water (marked with the letters B + C).
After standing in the freezer all night, the salt water did not freeze, but ice crystals formed in the glass. Fresh water turned to ice. While I was manipulating with cups and salt solutions, Vladik created his unplanned experiment.
He poured water, vegetable oil into a mug and discreetly put it in the freezer. 
Salt water in the freezer did not freeze, but what happens if you sprinkle salt on the ice? Let's check.
Experience with ice and salt
Take two ice cubes. Sprinkle one of them with salt, and leave the second for comparison. 
We froze a big icicle and sprinkled it with salt, took brushes and 
Our experienced creativity unites the whole family, so Makarushkin's pen got into the camera lens!
Makar and Vlad are very everyone loves to freeze
. Sometimes there are completely unexpected items in the freezer.
I dreamed of doing this experience since childhood, but my mother did not have a fur coat, and many

How great it is to be an adult, to have a fur coat and do all sorts of children's experiments!
Your Galina Kuzmina
The table shows the thermophysical properties of a solution of calcium chloride CaCl 2 depending on temperature and salt concentration: specific heat of the solution, thermal conductivity, viscosity of aqueous solutions, their thermal diffusivity and the Prandtl number. The concentration of salt CaCl 2 in solution is from 9.4 to 29.9%. The temperature at which the properties are given is determined by the salt content of the solution and ranges from -55 to 20°C.
calcium chloride CaCl 2 may not freeze up to minus 55°С
. To achieve this effect, the concentration of salt in the solution should be 29.9%, and its density will be 1286 kg/m 3 .
With an increase in the salt concentration in a solution, not only its density increases, but also such thermophysical properties as the dynamic and kinematic viscosity of aqueous solutions, as well as the Prandtl number. For instance, dynamic viscosity of CaCl 2 solution
with a salt concentration of 9.4% at a temperature of 20°C is 0.001236 Pa s, and with an increase in the concentration of calcium chloride in the solution to 30%, its dynamic viscosity increases to a value of 0.003511 Pa s.
It should be noted that the temperature has the strongest influence on the viscosity of aqueous solutions of this salt. When a solution of calcium chloride is cooled from 20 to -55°C, its dynamic viscosity can increase by 18 times, and kinematic by 25 times.
Given the following thermophysical properties of CaCl 2 solution
:
- , kg / m 3;
- freezing point °С;
- dynamic viscosity of aqueous solutions, Pa s;
- Prandtl number.
ÑаÑÑолÑемпеÑаÑÑÑа
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · α α ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ ñ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð δ¶¶Ð Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð Ð δÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð δ °ÑÑвоÑа ÑвелиÑиÑÑÑ. Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ññμ¼ññññññ ° ° ° ° ° °ñññ °ñññ °ññ °ñññ Ð ÐμÐ Ð Ð Ð Ð Ð Ðμ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ »Ð ²Ðððð ооо²²²²²² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ²Ð Ð Ð ² ÐÑÐ »Ð¸ ÑоÐ'ÐμÑжР° ниÐμ Ñол и в ÑÐ ° ÑÑоР»Ðμ мÐμнÑÑÐμ, ÑÐμм ÑÑÐμÐ ± ÑÐμмоÐμ Ð'л Ñ ÑвÑÐμкÑиÑÐμÑкого ÑÐ ° ÑÑвоÑÐ °, воÐ'Ð ° ÑÑкоÑÐ¸Ñ Ð¾Ð ±Ñазование кÑиÑÑаллов лÑда пÑи оÑлажденÑÑаѸлда пÑи оÑлажденÑÑаиоиии Running ñðμμðððμð½ððÐμÐμРРо Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ¸ÑÐ¸Ð²Ð°ÐµÑ ÐºÐ¾Ð½ÑенÑÑаÑÐ¸Ñ Ñоии оÑÑалÑном ÑÑÑаÑÐ¸Ñ Ñои оÑÑалÑном ÑÑÑаÑÐ¸Ñ Ñои оÑÑалÑном ÑÑÑаÑиÑÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ñ ññ ñ ñ Ð ° Ð ° ñ °
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ñÐ ññ ñ ñ ñ ññ ° ñ ° Ð ° С Ð Ð'Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð μ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð μñ ÐÐ ° мÐμÑÐ · Ð ° ниÐμ ÑвÑÐμкÑиÑÐμÑкого ÑÐ ° ÑÑвоÑÐ ° â ÑÑо пÑоÑÐμÑÑ Ñ ÑÑÐ ° ÑÑиÐμм ÑкÑÑÑой ÑÐμпР»Ð¾ÑÑ, поÑÑÐ¾Ð¼Ñ ÑÐμмпÐμÑÐ ° ÑÑÑÐ ° оÑÑÐ ° ÐμÑÑÑ Ð½ÐμиР· менной. Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ Ð ñ °Ð Ð Ð Ð Ðμ ñ ñ ² Ð ñ Ð Ð Ð ² Resume. Ð Ð · ñ ñ ñ ñ ñ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñ ññÐ Ð ° Ð ° Ð Ð Ð Ð'Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° ¸ поÑÑоÑнной ÐÐ ° ннÑй пÑоÑÐμÑÑ Ð¿ÑимÐμнÑÐμÑÑÑ Ð² пР»Ð ° ÑÑинÑÐ ° ÑÑÑ Ð¸ÑпР° ÑиÑÐμл ÑÑ Ð¿Ñи ÑÑÐ ° нÑпоÑÑиÑовкÐμ пÑоÐ'ÑкÑов .
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð Ð ° Ð ° Ð Ð Ð Ð ° Ð ° Ð ° калÑÑÐ¸Ñ Ð¸ ÑлоÑида наÑÑиÑ. Ð Ð ° ÑÑÐ²Ð¾Ñ ÑÐ »Ð¾ÑиÐ'Ð ° кР° л ÑÑÐ¸Ñ Ð¿ÑÐμжÐ'Ðμ вÑÐμго иÑпоР»ÑÐ · ÑÐμÑÑÑ Ð² пÑомÑÑл ÐμннÑÑ Ð¿ÑоÑÐμÑÑÐ ° Ñ Ð¿ÑÐ ¸ замоÑаживании пÑодÑкÑов, ÑÑанении и Ñ.д. 17.8 °С. Ð ° Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð 55 °C Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · Ð (μ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ¿Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ñññððμμ'''ðððððððð °Ð²Ð°ÑÑÑоÑÑкий пÑодÑкÑам Ðð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÑÑÑонÑÐ°ÐºÑ ÑаÑÑооа ÑоÑлаждаемÑм пÑодÑкÑом.
Ð Ð ° ÑÑÐ²Ð¾Ñ ÑÐ »Ð¾ÑиÐ'Ð ° нР° ÑÑÐ¸Ñ (пиÑÐμвой Ñол и) нÐμ поÑÑÐ¸Ñ Ð¿Ð¸ÑÐμвÑÐμ пÑоÐ'ÑкÑÑ. оñððð Ð ° Ð °ñÐ Ð Ð Ð ðñððРРРРРРРРРРРРРРРРРРРРРРРРРРРРоÐμ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð Ð Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ Ð ° Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² РРРРизиÑелÑно в21.1 °С. Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð ² Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ·
At what temperature can sea water freeze?
But the main question has not yet been answered. We have already learned that salt slows down the freezing of water, the sea will be covered with a crust of ice not at zero, but at sub-zero temperatures. But how far should the thermometer readings go to minus so that the residents of the coastal regions do not hear the usual sound of the surf when they leave their homes?
To determine this value, there is a special formula, complex and understandable only for specialists. It depends on the main indicator - salinity level
. But since we have an average value for this indicator, can we also find the average freezing point? Oh sure.
If you do not need to calculate everything up to a hundredth, for a particular region, remember the temperature at -1.91 degrees
.
It may seem that the difference is not so great, only two degrees. But during seasonal temperature fluctuations, this can play a huge role where the thermometer falls at least 0. It would be only 2 degrees cooler, the inhabitants of the same Africa or South America could see ice near the coast, but alas. However, we do not think that they are very upset by such a loss.
Thermophysical properties of NaCl solution
The table shows the thermophysical properties of a solution of sodium chloride NaCl depending on temperature and salt concentration. The concentration of sodium chloride NaCl in solution is from 7 to 23.1%. It should be noted that when an aqueous solution of sodium chloride is cooled, its specific heat capacity changes slightly, the thermal conductivity decreases, and the viscosity of the solution increases.
Given the following thermophysical properties of NaCl solution
:
- solution density, kg/m 3 ;
- freezing point °С;
- specific (mass) heat capacity, kJ/(kg deg);
- thermal conductivity coefficient, W/(m deg);
- dynamic viscosity of the solution, Pa s;
- kinematic viscosity of the solution, m 2 /s;
- thermal diffusivity, m 2 /s;
- Prandtl number.
What is sea water made of?
How is the content of the seas different from fresh water? The difference is not so great, but still:
- Much more salt.
- Magnesium and sodium salts predominate.
- The density differs slightly, within a few percent.
- Hydrogen sulfide can form at depth.
The main component of sea water, no matter how predictable it may sound, is water. But unlike the water of rivers and lakes, it contains large amounts of sodium and magnesium chlorides
.
Salinity is estimated at 3.5 ppm, but to make it more clear - at 3.5 thousandths of a percent of the total composition.
And even this, not the most impressive figure, provides water not only with a specific taste, but also makes it undrinkable. There are no absolute contraindications, sea water is not a poison or a toxic substance, and nothing bad will happen from a couple of sips. It will be possible to talk about the consequences if a person is at least throughout the day. Also, the composition of sea water includes:
- Fluorine.
- Bromine.
- Calcium.
- Potassium.
- Chlorine.
- sulfates.
- Gold.
True, in percentage terms, all these elements are much less than salts.
States and types of water
Water on planet Earth can take three main states of aggregation: liquid, solid and gaseous, which can transform into different forms that simultaneously coexist with each other (icebergs in sea water, water vapor and ice crystals in clouds in the sky, glaciers and free-flowing rivers ).
Depending on the characteristics of the origin, purpose and composition, water can be:
- fresh;
- mineral;
- nautical;
- drinking (here we include tap water);
- rain;
- thawed;
- brackish;
- structured;
- distilled;
- deionized.
The presence of hydrogen isotopes makes water:
- light;
- heavy (deuterium);
- superheavy (tritium).
We all know that water can be soft and hard: this indicator is determined by the content of magnesium and calcium cations.
Each of the types and aggregate states of water we have listed has its own freezing and melting point.
Freezing Salt Water Video

 What do you know about the boiling point of water?
What do you know about the boiling point of water?

The temperature regime determines, first of all, the rate of the freezing process.
Temperature in the range of positive and negative values affects the rate of reactions, the solubility of compounds, the rate of dissolution, coagulation, as well as the concentration of undissociated ion pairs. There are several types of temperature in solutions: structural, freezing point. Crystallization start temperature (freezing point) - the temperature at which, as a result of cooling the solution, the formation of crystals begins. Freezing point depression ΔТз is the difference between the freezing point of a pure solvent and a solution. The freezing point of brine is always below the freezing point of pure water and depends on the concentration of dissolved salts. This dependence for brines can be expressed by the equation:
where TO
— coefficient of proportionality; WITH
is the concentration of the solute in the solution.
In less dilute solutions, the crystallization onset temperature is determined from the state diagram of the corresponding system. Since the freezing temperature of sea waters and highly mineralized natural brines will be different, we assume that this temperature should be calculated using different formulas.
We have made an approximation of the experimental data on the freezing points of solutions of table salt, sea water and natural brines used in the work. The dependences of freezing temperature changes in graphical and analytical forms are presented in Figures 41-43.
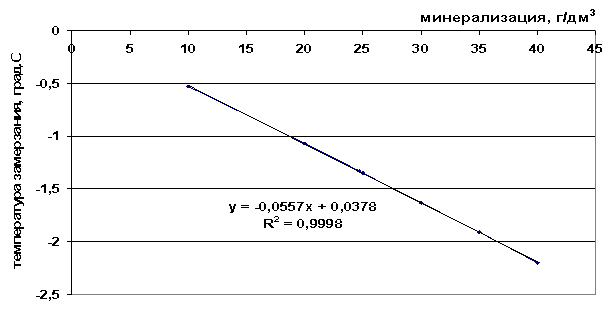
Rice. 41. The dependence of the freezing point on the salinity of a salt solution
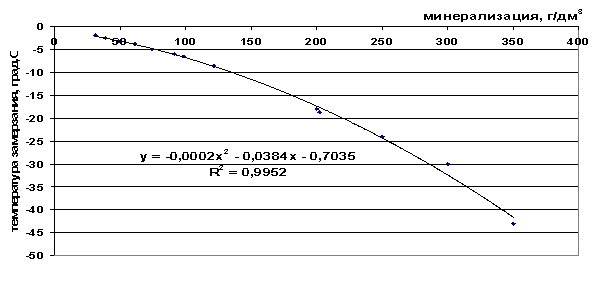
Rice. 42. Dependence of the freezing point of sea water on salinity
Rice. 43. Dependence of the freezing point of brine on salinity
From the presented freezing point values (Table 9), it can be seen that the freezing point decreases as the total salinity of the solution increases and as the number of components included in the frozen system increases - ΔТз(NaCl)
Table 9. Analysis of the constructed graphical dependencies
|
Сtot, g/dm 3 |
Freezing point, °С |
||
|
NaCl solution |
sea water |
||
|
t=8∙10 -5 M 2 -0.0945M+1.0595, |
0.0557M+0.0378, |
t=-2∙10 -4 M 2 -0.0384M-0.7035, |
|
*
R 2 - reliability of approximation
It is known that the freezing of individual salts from desalinated water occurs at different temperatures, for example, at a temperature of -2°C, calcium carbonate precipitates. At - 3.5 ° C sodium sulfate. When the temperature drops to -20 ° C, table salt precipitates, to -25.5-26 ° C magnesium chlorides, and at very low temperatures - 40-55 ° C, potassium and calcium chlorides precipitate. For negative temperatures, the process of formation of crystalline hydrates, which are unstable at temperatures below 0°C, is specific. For example, hydrohalite NaCl * 2H 2 O is formed at -0.15 ° C, MgCl 2 * 12H 2 O is stable at -15 ° C, and MgCl 2 * 8H 2 O is below 0 ° C, Na 2 CO 3 * 7H 2 O is formed only at -10°C. KCl crystallizes at 0°С in the form of KCl, at -6.6°С already two phases coexist - KCl and KCl*H 2 O, at -10.6°С only KCl*H 2 O precipitates. At negative temperatures, individual crystalline hydrates with the maximum possible number of molecules of crystallization water, according to the coordination numbers at a given value, and their mixtures (but not mixed crystals). An anomalous decrease in the freezing point of concentrated solutions should be noted.
We bring to your attention the journals published by the publishing house "Academy of Natural History"
At what temperature does water freeze? It would seem - the simplest question that even a child can answer: the freezing point of water at normal atmospheric pressure of 760 mmHg is zero degrees Celsius.
However, water (despite its extremely wide distribution on our planet) is the most mysterious and not fully understood substance, so the answer to this question requires a detailed and reasoned discussion.
- In Russia and Europe, the temperature is measured on the Celsius scale, the highest value of which is 100 degrees.
- The American scientist Fahrenheit developed his own scale with 180 divisions.
- There is another unit of temperature measurement - kelvin, named after the English physicist Thomson, who received the title of Lord Kelvin.
Why you shouldn't drink sea water
We have already briefly touched on this topic, let's look at it in a little more detail. Together with sea water, two ions enter the body - magnesium and sodium.
|
Sodium |
Magnesium |
|
Participates in maintaining the water-salt balance, one of the main ions along with potassium. |
The main effect is on the central nervous system. |
|
With an increase in the number Na |
Very slowly excreted from the body. |
|
All biological and biochemical processes are disturbed. |
An excess in the body leads to diarrhea, which aggravates dehydration. |
|
Human kidneys are not able to cope with so much salt in the body. |
Perhaps the development of nervous disorders, inadequate condition. |
It cannot be said that a person does not need all these substances, but needs always fit within certain limits. After drinking a few liters of such water, you will go too far beyond their limits.
However, today the urgent need for the use of sea water may arise only among the victims of shipwrecks.