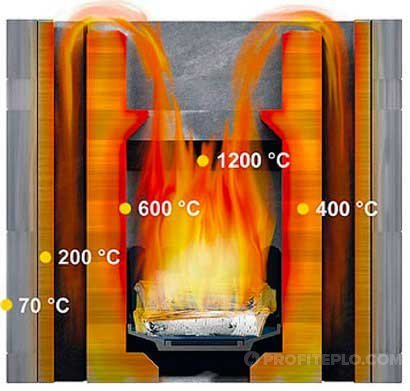
Factors affecting the combustion temperature
The temperature of burning wood in a stove depends not only on the type of wood. Significant factors are also the moisture content of firewood and the traction force, which is due to the design of the thermal unit.
Influence of humidity
In freshly cut wood, the moisture index reaches from 45 to 65%, on average - about 55%. The combustion temperature of such firewood will not rise to the maximum values, since the thermal energy will be spent on the evaporation of moisture. In accordance with this, the heat transfer of the fuel is reduced.
In order for the required amount of heat to be released during the combustion of wood, three ways are used
:
- almost twice as much freshly cut firewood is used for space heating and cooking (this translates into higher fuel costs and the need for frequent maintenance of the chimney and gas ducts, in which a large amount of soot will settle);
- freshly cut firewood is pre-dried (the logs are sawn, split into logs, which are stacked under a canopy - it takes 1-1.5 years for natural drying to 20% humidity);
- dry firewood is purchased (financial costs are offset by the high heat transfer of the fuel).
The calorific value of birch firewood from freshly cut wood is quite high. Freshly cut ash, hornbeam and other hardwood fuels are also suitable for use.
Influence of air supply
By limiting the supply of oxygen to the furnace, we lower the combustion temperature of the wood and reduce the heat transfer of the fuel. The duration of combustion of the fuel load can be increased by closing the damper of the boiler unit or stove, but fuel savings result in low combustion efficiency due to suboptimal conditions. To the wood burning in an open-type fireplace, air enters freely from the room, and the intensity of draft depends mainly on the characteristics of the chimney.
The simplified formula for the ideal combustion of wood is
:
C + 2H2 + 2O2 = CO2 + 2H2O + Q (heat)
Carbon and hydrogen are burned when oxygen is supplied (left side of the equation), resulting in heat, water and carbon dioxide (right side of the equation).
In order for dry wood to burn at maximum temperature, the volume of air that enters the combustion chamber must reach 130% of the volume required for the combustion process. When the air flow is blocked by dampers, a large amount of carbon monoxide is formed, and the reason for this is the lack of oxygen. Carbon monoxide (unburnt carbon) goes into the chimney, while the temperature in the combustion chamber drops and the heat transfer of firewood decreases.
An economical approach when using a solid fuel wood-fired boiler is to install a heat accumulator that will store excess heat generated during fuel combustion in the optimal mode, with good traction.
With wood-burning stoves, you won’t be able to save fuel like that, since they directly heat the air. The body of a massive brick oven is capable of accumulating a relatively small part of the thermal energy, while for metal stoves, excess heat goes directly into the chimney.
If you open the blower and increase the draft in the furnace, the combustion intensity and heat transfer of the fuel will increase, but the heat loss will also increase. With the slow combustion of firewood, the amount of carbon monoxide increases and heat transfer decreases.
We build a Russian bath according to the mind
Views: 3 082 As a rule, the main source of heat received for the needs of soaring in the bath is burning firewood.
But first, let's briefly touch on the question of the structure of wood as a fuel.
Wood is a combination of hydrocarbon compounds (polysaccharide polymers) of cellulose, hemicellulose and lignin.
It is capable of burning and forms explosive mixtures with air. Carbon monoxide, when burned, produces a blue flame. Carbon monoxide is highly toxic. Inhalation of air with a carbon monoxide concentration of 0.4% is fatal to humans.
Info
Standard gas masks do not protect against carbon monoxide, so special filters or oxygen isolation devices are used in case of fires.
Sulphur dioxide
Sulfur dioxide (SO 2 ) is a combustion product of sulfur and sulfur compounds. A colorless gas with a characteristic pungent odor. Relative density of sulfur dioxide = 2.25. The density of this gas at T = 0 0 C and p = 760 mm Hg is 2.9 kg/m 3 , that is, it is much heavier than air.
Let us briefly consider the properties of the main combustion products.
Carbon dioxide
Carbon dioxide or carbon dioxide (CO 2) is a product of the complete combustion of carbon. Has no smell and color. Its density in relation to air = 1.52. The density of carbon dioxide at a temperature T \u003d 0 0 C and at normal pressure p \u003d 760 millimeters of mercury (mm Hg) is 1.96 kg / m 3 (air density under the same conditions is ρ \u003d 1.29 kg / m 3).
Important
Carbon dioxide is highly soluble in water (at T = 15 0 C, one liter of gas dissolves in one liter of water). Carbon dioxide does not support combustion of substances, with the exception of alkali and alkaline earth metals
The combustion of magnesium, for example, occurs in an atmosphere of carbon dioxide according to the equation:
CO 2 +2 Mg \u003d C + 2 MgO.
The toxicity of carbon dioxide is negligible.
Views: 3 317
As a rule, the main source of heat received for the needs of soaring in the bath is burning firewood.
Understanding what the process of burning wood is like and the ability to control the amount of heat extracted during this and its most efficient use, allows you to consciously make a choice in favor of one or another model of a sauna stove.
So, let's consider the chemical and physical foundations of the process of burning wood fuel, which occurs in the firebox of any sauna stove.
But first, let's briefly touch on the question of the structure of wood as a fuel.
Wood is a combination of hydrocarbon compounds (polysaccharide polymers) of cellulose, hemicellulose and lignin.
They only heat up due to the heat of combustion of carbon C and hydrogen H released from the heated wood. Or, to put it another way, these gases play a negative role in combustion. They cool the combustion zone, prevent the completeness of the oxidation reactions of the combustible components of wood until they are converted into the final products CO2 and H2O, reduce the heating of the furnace, and ultimately determine the heat content of the combustion products of the fuel.
So let's draw the line.
We have considered the physical and chemical basis of the process of combustion of hydrocarbon fuel, which is wood.
It was determined that the main purpose of burning wood in a stove is the completeness of their combustion and the maximum use of the released thermal and radiation energy.
At this stage, the tree actively absorbs heat from the outside. There is no combustion process.
At temperatures of 150-275ºС, the process of decomposition of the original wood structure into simpler solid, liquid and gaseous components (carbon monoxide CO, carbon dioxide CO2, methane CH4, wood alcohol (methanol) CH3OH, acetic acid CH3COOH, creosote-a mixture of phenols and aromatic hydrocarbons) begins. ). Wood continues to actively absorb heat. There is no combustion.
At temperatures of 275-450ºС, the process of active decomposition and simplification of the wood structure begins with the rapid release of heat, gaseous fuels and self-heating of wood. The breakdown of cellulose and lignin begins.
Ideally, only nitrogen N2 should be emitted into the atmosphere through the chimney, as the main component of the air supplied to the furnace furnace along with oxygen, but not taking part in combustion, carbon dioxide CO2 and water vapor H2O.
As mentioned earlier, the products of the reaction of complete combustion of firewood are carbon dioxide CO2 from the combustion of carbon and water vapor H2O from the combustion of hydrogen.
As the ballast gases, water vapor of the H2O fuel released by the wood during heating, nitrogen N2, and also excess air act as ballast gases.
Combustion reaction products and ballast gases do not take part in combustion.
Release of substances Incomplete combustion of wood
Safety
- Before starting the experiment, put on protective gloves and goggles.
- Do the experiment on a tray.
- Keep a container of water nearby during the experiment.
- Remove gloves before lighting the torch.
General safety rules
- Avoid getting chemicals in your eyes or mouth.
- Do not allow people without goggles, as well as small children and animals, to the experiment site.
- Keep the experimental kit out of the reach of children under 12 years of age.
- Wash or clean all equipment and accessories after use.
- Make sure all reagent containers are tightly closed and properly stored after use.
- Make sure all disposable containers are properly disposed of.
- Use only the equipment and reagents supplied in the kit or recommended in the current instructions.
- If you have used a food container or experiment utensils, discard them immediately. They are no longer suitable for food storage.
First Aid Information
- If reagents come into contact with eyes, rinse eyes thoroughly with water, keeping eyes open if necessary. Seek immediate medical attention.
- If swallowed, rinse mouth with water, drink some clean water. Don't induce vomiting. Seek immediate medical attention.
- In case of inhalation of reagents, remove the victim to fresh air.
- In case of skin contact or burns, flush the affected area with plenty of water for 10 minutes or longer.
- If in doubt, consult a doctor immediately. Take a chemical reagent and a container from it with you.
- In case of injury, always consult a doctor.
Special combustion modes
Smoldering
Smoldering is a special type of slow combustion, which is maintained by the heat released in the reaction of oxygen and hot condensed matter directly on the surface of the substance and accumulated in the condensed phase. A typical example of smoldering is a lit cigarette. During smoldering, the reaction zone slowly spreads through the material. The gas-phase flame is not formed due to the insufficient temperature of the gaseous products or it goes out due to large heat losses from the gas phase. Smoldering is commonly seen in porous or fibrous materials. Smoldering can be a great danger during a fire, since incomplete combustion releases substances that are toxic to humans.
Solid state combustion
Infrared gas stove with porous matrices as heating elements
In mixtures of inorganic and organic powders, autowave exothermic processes can occur, which are not accompanied by noticeable gas evolution and form only condensed products. At intermediate stages, gaseous and liquid phases can be formed, which, however, do not leave the burning system. Examples of reacting powders are known in which the formation of such phases has not been proven (tantalum-carbon). Such modes are called solid phase combustion, the terms are also used gasless combustion and solid flame combustion. These processes have found practical application in the technologies of self-propagating high-temperature synthesis (SHS) developed under the guidance of A. G. Merzhanov.
Combustion in a porous medium
If the initial combustible mixture passes through a porous medium, for example, a ceramic matrix, then during its combustion part of the heat is spent on heating the matrix. The hot matrix, in turn, heats up the initial mixture. Thus, part of the heat of the combustion products is recovered, which makes it possible to use lean mixtures (with a low fuel excess ratio), which do not burn without heat recirculation.Porous combustion technologies (also referred to as filtration combustion in the domestic literature) can reduce emissions of harmful substances and are used in gas infrared stoves, heaters, and many other devices.
Flameless burning
Unlike conventional combustion, when a luminous flame zone is observed, it is possible to create conditions for flameless combustion. An example is the catalytic oxidation of organic substances on the surface of a suitable catalyst, for example, the oxidation of ethanol on platinum black. However, the term "flameless combustion" is not limited to the case of surface catalytic oxidation, but refers to situations in which the flame is not visible to the naked eye. Therefore, combustion modes in radiation burners or some modes of exothermic decomposition of ballistic powders at low pressure are also called flameless. Flameless oxidation, a special way of organizing low-temperature combustion, is one of the promising directions in the creation of low-emission combustion chambers for power plants.
Literature
- Gaydon A. Spectroscopy and combustion theory. — M.: Publishing house of foreign literature, 1950. - 308 p.
- Khitrin L. N. Physics of combustion and explosion. — M.: Publishing House of Moscow University, 1957. - 452 p.
- Shchelkin K.I., Troshin Ya.K. Gas dynamics of combustion. — M.: Publishing House of the Academy of Sciences of the USSR, 1963. - 254 p.
- Lewis B., Elbe G. Combustion, flame and explosions in gases. 2nd ed. Per. from English. ed. K. I. Shchelkin and A. A. Borisov. — M.: Mir, 1968. - 592 p.
- Pokhil P. F., Maltsev V. M., Zaitsev V. M. Methods for studying combustion and detonation processes. — M.: Nauka, 1969. - 301 p.
- Novozhilov B.V. Unsteady combustion of solid rocket propellants. — M.: Nauka, 1973. - 176 p.
- Lawton J., Weinberg F. Electrical aspects of combustion. — M.: Energy, 1976. - 296 p.
- Zeldovich Ya. B., Barenblatt G. I., Librovich V. B., Makhviladze G. M. Mathematical theory of combustion and explosion. — M.: Nauka, 1980. - 479 p.
- (English)
- (English)
- (English)
- (English)
- (English)
- (English)
heterogeneous combustion
Heterogeneous processes, as opposed to homogeneous, in chemistry and physics are called processes occurring in heterogeneous systems, that is, systems containing more than one phase (for example, gas and liquid), as well as processes occurring at the phase boundary. In combustion research, the term heterogeneous combustion used for systems in which the fuel and oxidizer are initially in different phases, even if in the process the fuel is vaporized and the chemical reactions themselves occur in the gas phase. A typical example is the combustion of coal in air, in which carbon can react with oxygen on the surface of the coal particles to form carbon monoxide. Subsequently, carbon monoxide can burn out in the gas phase and form carbon dioxide, and in some modes, the fuel can evaporate from the surface of the particles and oxidize as gaseous carbon in the gas phase. Despite the difference in mechanisms, all these regimes are formally related to heterogeneous combustion.
Heterogeneous combustion is extremely important in practical applications of combustion. Most fuels are more convenient to store and transport in liquid form (including liquefied natural gas)
Work processes in furnaces, internal combustion engines, diesel engines, air-jet engines, liquid rocket engines are heterogeneous combustion, and optimization of the process of evaporation and mixing of fuel and oxidizer for their supply to the combustion chamber is an important part of optimizing the entire combustion process in workers. systems.
Almost all fires are also heterogeneous combustion, but household gas explosions are homogeneous combustion, since both the fuel and the oxidizer are initially gases.
To improve the energy characteristics of solid fuels, metals can be added to them. Such fuels can be used, for example, for high-speed submarine torpedoes, since pure aluminum burns well in water. The combustion of aluminum and other metals occurs according to a heterogeneous mechanism.
What is the combustion process
Combustion is a process at the turn of physics and chemistry, which consists in the transformation of a substance into a residual product. At the same time, thermal energy is released in large quantities. The combustion process is usually accompanied by the emission of light, which is called a flame. Also, during the combustion process, carbon dioxide is released - CO 2, an excess of which in an unventilated room can lead to headaches, suffocation and even death.
For the normal course of the process, a number of mandatory conditions must be met.
First, combustion is possible only in the presence of air. Impossible in a vacuum.
Secondly, if the area in which combustion occurs is not heated to the ignition temperature of the material, then the combustion process will stop. For example, the flame will go out if a large log is immediately thrown into a freshly fired oven, preventing it from warming up on small wood.
Thirdly, if the subjects of combustion are damp and emit liquid vapors, and the combustion rate is still low, the process will also stop.

Notes
- I.N. Zverev, N. N. Smirnov. Gas dynamics of combustion. — M.: Moscow Publishing House. un-ta., 1987. - S. 165. - 307 p.
- Combustion is sometimes defined as the reaction between an oxidizer and a fuel. However, combustion processes include, for example, both the combustion of monomolecular fuels and the decomposition of ozone, when chemical energy is stored in chemical bonds in one substance.
- ↑ Burning //: / Ch. ed. A. M. Prokhorov. - 3rd ed. — M. : Soviet encyclopedia, 1969-1978.
- . Chemical Encyclopedia. Retrieved 16 September 2013.
- (English) 1. U.S. Energy Information Administration (EIA). Retrieved 4 February 2014.
- Mallard E., Le Chatelier H. L. Thermal model for flame propagation // Annals of Mines. - 1883. - Vol. 4. - P. 379.
- , With. eight.
- Michelson V. A. On the normal rate of ignition of explosive gas mixtures. - Sobr. op. M.: New agronomist, 1930, v. 1
- Burke S.P., Schumann T.E.W. Diffusion flames // Industrial & Engineering Chemistry. - 1928. - Vol. 20, No. 10. - P. 998-1004.
- , With. 9.
- Frank-Kamenetsky D. A. Temperature distribution in a reaction vessel and stationary theory of thermal explosion // Journal of Physical Chemistry. - 1939. - T. 13, No. 6. - S. 738-755.
- Zeldovich Ya. B., Frank-Kamenetsky D. A. Theory of Thermal Flame Propagation // Journal of Physical Chemistry. - 1938. - V. 12, No. 1. - S. 100-105.
- Belyaev A.F. On the combustion of explosives // Journal of Physical Chemistry. - 1938. - T. 12, No. 1. - S. 93-99.
- Zeldovich Ya. B. On the theory of combustion of gunpowder and explosives // Journal of Experimental and Theoretical Physics. - 1942. - T. 12, No. 1. - S. 498-524.
- Zeldovich Ya. B. On the theory of detonation propagation in gaseous systems // Journal of Experimental and Theoretical Physics. - 1940. - T. 10, no. 5. - S. 542-568.
- von Neumann J. Theory of detonation waves. Progress Report to the National Defense Research Committee Div. B, OSRD-549 (April 1, 1942. PB 31090) // Theory of detonation waves. - John von Neumann: Collected Works, 1903-1957. - Oxford: Pergamon Press, 1963. - Vol. 6. - P. 178-218. - ISBN 978-0-08-009566-0.
- , With. 26.
- , With. 659.
- , With. 9.
- , With. 206.
- , With. 686.
- , With. eight.
- ↑ , p. 10.
- , With. 578.
- , With. 49.
- , With. 60.
- , With. 183.
- , With. 9.
- , With. 12.
- . Prof. Burcat's Thermodynamic Data. Retrieved 13 August 2013.
- . eLearning@CERFACS. Retrieved 13 August 2013.
- . Retrieved 13 August 2013.
- , With. 25.
- , With. 95.
- , With. 57.
- , With. 66.
- , With. 187.
- , With. 193.
- , With. 200.
- .
- , With. one.
- , With. 132.
- , With. 138.
- .
- . Cnews. Retrieved 19 August 2013.
- , With. 10.
- Pokhil P.F. Doctoral dissertation. Institute of Chemical Physics of the Academy of Sciences of the USSR. 1953
- , With. 177.
- , With. 24.
- ↑
- Leipunsky O.I. Doctoral dissertation. Institute of Chemical Physics of the Academy of Sciences of the USSR. 1945
- Leipunsky O.I. To the question of the physical foundations of the internal ballistics of rocket projectiles // Theory of combustion of gunpowder and explosives / Ed. editors: O. I. Leipunsky, Yu. V. Frolov. — M. : Science, 1982. - S. 226-277.
- , With. 26.
- Zeldovich Ya. B. On the theory of combustion of gunpowder and explosives // Journal of Experimental and Theoretical Physics. - 1942. - T. 12, No. 1. - S. 498-524.
- , With. 40.
- Ohlemiller T.J. (English). SFPE Handbook of Fire Protection Engineering, 3rd Edition. NIST (2002). Retrieved 15 August 2013.
- Merzhanov A. G., Mukasyan A. S. Solid flame combustion. — M.: Torus Press. — 336 p. - 300 copies. - ISBN 978-5-94588-053-5.
- Institute of Structural Macrokinetics and Problems of Materials Science RAS. . Retrieved 20 August 2013.
- . Big encyclopedia of oil and gas. Retrieved 31 August 2013.
- , With. 23.
Classification of combustion types
According to the speed of movement of the mixture, combustion is divided into slow burning (or deflagration) and detonation combustion (detonation).The deflagration combustion wave propagates at subsonic speed, and the initial mixture is heated mainly by thermal conductivity. The detonation wave travels at supersonic speed, while the chemical reaction is supported by the heating of the reactants by the shock wave and, in turn, supports the steady propagation of the shock wave. Slow combustion is subdivided into laminar and turbulent according to the nature of the mixture flow. In detonation combustion, the flow of products is always turbulent. Under certain conditions, slow combustion can turn into detonation (eng. DDT, deflagration-to-detonation transition).
If the initial components of the mixture are gases, then combustion is called gas-phase (or homogeneous). In gas-phase combustion, an oxidizing agent (usually oxygen) interacts with a fuel (for example, hydrogen or natural gas). If the oxidizer and fuel are premixed at the molecular level, then this mode is called premixed combustion. If the oxidizer and fuel are separated from each other in the initial mixture and enter the combustion zone through diffusion, then combustion is called diffusion.
If the oxidizer and fuel are initially in different phases, then combustion is called heterogeneous. As a rule, in this case, the oxidation reaction also proceeds in the gas phase in the diffusion mode, and the heat released in the reaction is partially spent on thermal decomposition and evaporation of the fuel. For example, coal or polymers in the air burn according to this mechanism. In some mixtures, exothermic reactions in the condensed phase may occur to form solid products without significant gas evolution. This mechanism is called solid-phase combustion.
There are also such special types of combustion as smoldering, flameless and cold-flame combustion.
Combustion, or nuclear combustion, is called thermonuclear reactions in stars, in which the nuclei of chemical elements are formed in the processes of stellar nucleosynthesis.
Thermal characteristics of wood
Wood species differ in density, structure, quantity and composition of resins. All these factors affect the calorific value of wood, the temperature at which it burns, and the characteristics of the flame.
Poplar wood is porous, such firewood burns brightly, but the maximum temperature indicator reaches only 500 degrees. Dense wood species (beech, ash, hornbeam), burning, emit over 1000 degrees of heat. Birch indicators are somewhat lower - about 800 degrees. Larch and oak flare up hotter, giving out up to 900 degrees of heat. Pine and spruce firewood burns at 620-630 degrees.
The quality of firewood and how to choose the right one
Birch firewood has the best ratio of heat efficiency and cost - it is not economically profitable to heat with more expensive species with high combustion temperatures.
Spruce, fir and pine are suitable for making fires - these softwoods provide relatively moderate heat. But it is not recommended to use such firewood in a solid fuel boiler, in a stove or fireplace - they do not emit enough heat to effectively heat the home and cook food, they burn out with the formation of a large amount of soot.
Fuel from aspen, linden, poplar, willow and alder is considered low-quality firewood - porous wood emits little heat during combustion. Alder and some other types of wood "shoot" embers in the process of burning, which can lead to a fire if firewood is used to fire an open fireplace.
When choosing, you should also pay attention to the degree of moisture content of the wood - raw firewood burns worse and leaves more ash
What determines the efficiency of combustion
Combustion efficiency is an indicator that is determined by thermal energy, which does not “fly away into the chimney”, but is transferred to the furnace, heating it. This figure is influenced by several factors.
First of all, it is the integrity of the furnace design. Cracks, cracks, excess ash, a dirty chimney and other problems make combustion inefficient.
The second important factor is the density of the tree. Oak, ash, pear, larch and birch have the highest density. The smallest - spruce, aspen, pine, linden. The higher the density, the longer the piece of wood will burn, and therefore the longer it will release heat.
Large pieces of wood will not immediately catch fire. It is necessary to kindle a fire, starting with small branches. They will give coals that will provide the necessary temperature to ignite the wood loaded into the furnace in larger portions.
Ignition products, especially in the barbecue, are not recommended, as they emit substances harmful to humans when burned. Too much ignition agent in a closed firebox can cause an explosion.

But still, how tar is formed in furnaces
The main element of which wood, brown or hard coal is composed, is carbon. Water makes up 20-35% of the weight of wood, and potassium, magnesium, sodium and other elements do not exceed 1-3% of the weight and remain mainly in ash residues, taking a minimal part in the formation of tar.
It is carbon that burns in furnaces. And if in simple solid fuel boilers there are fairly simple processes that are easy to manage, but difficult to automate, then in pyrolysis furnaces it is the aforementioned process of dry distillation of wood that can occur much more often.
Under the influence of high temperature and insufficient oxygen, thermal decomposition of wood occurs: wood gas is released, which consists of carbon monoxide, hydrogen, nitrogen (located in primary air), as well as the main heroes of the occasion - hydrocarbons of carbon compounds with nitrogen, oxygen, hydrogen (for example, methane, propane, acetylene). Further, due to the secondary air injection into the afterburning chamber of the boiler, the released gases are burned. With incomplete combustion of these gases, namely hydrocarbons, a chemical reaction occurs, during which tar is formed.
With incomplete combustion of these gases, namely hydrocarbons (methane, propane, etc.), instead of combustion, a chemical reaction occurs, during which tar is formed.
Pyrolysis boilers are known for their high efficiency, their efficiency, they are able to use the energy of chemical bonds of wood, carbon by 97-98%. If fuel oil, tar is formed in the boiler, then this means that you should forget about efficiency, and your boiler is configured, assembled or installed incorrectly!
The main reason for the appearance of tar in the chimney is an insufficient amount of oxygen supplied to the combustion chamber, which leads to a decrease in the temperature at which the process should take place.
You can also identify reasons such as improper assembly and layout, low-powered blower (pump) of the boiler, voltage drop in the network, insufficiently high chimney, damp firewood. You should also not be too economical: air supply below a certain level can stretch the combustion process (pyrolysis) in the boiler for a longer period of time, but will lead to the formation of tar. And this is fraught not only with regular cleaning of the chimney, but also with the failure of the boiler and combustion chamber.
How to deal with tar if it has already begun to form?
-
Raising the combustion temperature. This can be done by increasing the air supply and using drier wood.
-
Changing the geometry, length of the chimney, gas ducts. This should reduce gas resistance, improve traction, and thus increase air supply without increasing the power of the supercharger (pump).
-
Increasing the combustion temperature by adjusting the pump output or adding drier wood at the end of the fire. This will help burn out the tar that has managed to form in the chimney.
If a significant amount of tar has appeared in the chimney, it should first be cleaned with a chemical or antiquated method. And only then change the system configuration.
A significant rise in temperature and subsequent ignition of tar in the chimney can lead to a roof fire or other catastrophic consequences. Tar is flammable, so you should be extremely careful.
A tar fire will clear the chimney, but can be a fire hazard |
The theory is also quite popular that the formation of tar depends on the type of wood. On the net you can find a lot of information that tar is formed only from the firebox with coniferous or certain types of wood, and you can fight it by burning birch firewood. Here it is worth remembering that our ancestors extracted tar from birch bark, laying it in a closed pot with a hole in the bottom and heating it up. And the burning of tar in the chimney when changing fuel can be explained not by a different chemical composition, but by a better degree of drying or a higher combustion temperature. So the association of tar with tree resin is just a delusion.
Let's summarize. Tar in a chimney, fireplace, chimney is not a diagnosis, it is just a symptom. How to find and cure the problem - our next publications will tell you.
For more information, we advise you to contact Waterstore specialists.
How man mastered fire
Fire was known to people who lived in the Stone Age. People have not always been able to make fire on their own. The first acquaintance of a person with the combustion process, according to scientists, occurred empirically. Fire, extracted from a forest fire or won from a neighboring tribe, was guarded as the most precious thing that people had.
Over time, a person noticed that some materials have the most burning properties. For example, dry grass or moss can be ignited by just a few sparks.
After many years, again empirically, people learned to extract fire using improvised means. Historians call the first “lighter” of a person tinder and flint, which, when they hit each other, gave sparks. Later, mankind learned to extract fire with the help of a twig placed in a special recess in the wood. The ignition temperature of the tree was achieved by intensive rotation of the end of the twig in the recess. Many Orthodox communities continue to use these methods today.

Much later, in 1805, the French chemist Jean Chancel invented the first matches. The invention gained enormous distribution, and a person could already confidently extract fire if necessary.
The development of the combustion process is considered the main factor that gave impetus to the development of civilization. Moreover, combustion will remain such a factor in the near future.