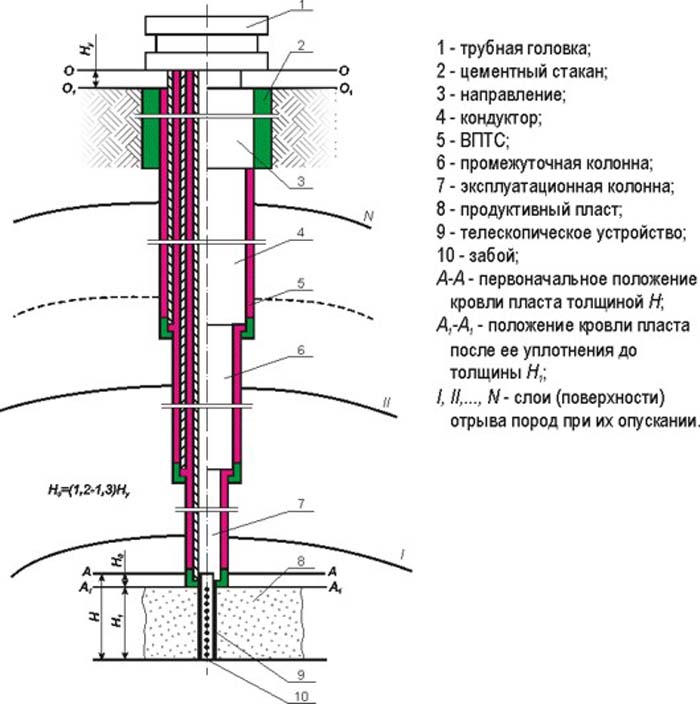
The role of dissolved oxygen DO
Despite the fact that the respiratory system of aquatic inhabitants is arranged differently than that of the inhabitants of the land-air environment, they still need the same substances. First of all, we are talking about oxygen, which plays an important role in the life of the vast majority of organisms. And if we extract it from the atmosphere, where its share is more or less stable and is about 21%, then the inhabitants of rivers, seas and oceans are highly dependent on how much oxygen is contained in the water in their habitat. In addition to fish, plants also need oxygen. However, its production is usually higher than consumption levels, so this should not be a concern.
How to find out the composition of air
The gaseous mixture that we breathe has long been interpreted by various philosophical schools as a unique substance that gives life. The Indians called it prana, the Chinese called it qi.
In the middle of the 18th century, the brilliant French naturalist A. Lavoisier, with his chemical experiments, debunked an erroneous scientific hypothesis about the existence of a special substance - phlogiston. It allegedly contained particles of an unknown energy that gives life to everything that exists on Earth. Lavoisier proved that the composition and properties of air are determined by the presence of two main gases: oxygen and nitrogen. They account for more than 98%. The remainder includes carbon dioxide, hydrogen, inert elements and industrial waste impurities such as gaseous oxides of nitrogen or sulfur. The study of the properties of the components of the atmosphere served as an incentive for humans to use this gaseous mixture in various branches of technology and in everyday life.
some chemistry
As you know, water (it is also hydrogen oxide) is a binary inorganic compound. Water is formed as a result of the combination of two hydrogen atoms and one oxygen atom. Formula - H2Oh
From this it is clear that without oxygen the existence of such a substance as water is impossible. And its number is constantly decreasing. Oxygen in water is consumed biologically (they breathe aquatic organisms), biochemically (this includes the respiration of bacteria, as well as the decomposition of organic matter) and chemically (as a result of oxidation).
But if oxygen is consumed, then its loss must be compensated.
The average flight altitude of a passenger aircraft is 9-12 thousand meters.
The air in this part of the atmosphere is already significantly rarefied, and its temperature is below minus 45 0C. Nevertheless, the conditions in the cabin of the liner are always relatively comfortable. This is due not only to good insulation, but also to a complex system that allows you to convert the air overboard into breathable. And yet, if you look, the conditions created do not quite correspond to the usual earthly atmosphere.
At the very beginning of the era of aviation, aircraft were made completely sealed, but due to the strong pressure difference inside and outside the aircraft, the metal was stretched, which led to the destruction of the structure. Therefore, at the moment, the cabin is maintained at a lower pressure than what corresponds to the level of the airport.
However, too little air compression in the cabin can cause severe discomfort to passengers by reducing the force with which oxygen presses on the walls of blood vessels. An altitude of 2500 meters corresponds to the upper pressure point, when the blood is still normally saturated with oxygen, and the person does not experience headaches, shortness of breath, nausea and severe fatigue. Most often, during the flight, pressure is maintained corresponding to an altitude of 1300-1800 meters, that is, 600-650 millimeters of mercury.
When inhaling, an adult consumes an average of 0.0005 cubic meters of air. We perform an average of 18 respiratory cycles per minute, processing 0.009 cubic meters of air during this time. It seems to be a little.But the interior of the liner is designed for an average of 600 passengers, therefore, they all need 5.4 cubic meters of air per minute. The air is gradually “polluted”, the oxygen content in it drops and after a while it will become simply impossible to breathe. Consequently, for the comfort (and in general to maintain life) of passengers, an influx of fresh air into the cabin is necessary.
All modern aircraft are equipped with a system that simultaneously provides the cabin with oxygen and keeps the engine running, since the fuel in it is burned only when oxidized by oxygen. When air from the atmosphere enters the internal circuit of the engine, it is highly compressed and, due to this, heats up. Further, from one of the compressor stages (a device for compressing gaseous substances), air is taken already for the passenger compartment. In this case, the intake takes place before mixing with the fuel, therefore it is absolutely harmless and clean, but just in case, it is still driven through the filters.
Aircraft engine diagram
The temperature of the air heated in the engine is about 500 0C. Therefore, before entering the cabin, it is sent to a radiator (a device for dissipating heat), where it is cooled, and then enters a turbo-cooler, rotating the aircraft turbine due to its expansion. The energy of the air decreases, the temperature drops to 20C.
As a result, two different air flows enter the cabin: hot, which did not pass through the turbo-cooler, and cold, which passed through it. The pilot controls the temperature in the cabin by mixing hot and cold air in the required proportions.
RIA Novosti illustration. Alina Polyanina
Adjusting the air temperature in the cabin
The main disadvantage of the system is that the air entering the cabin is too dry. Rarefied in the atmosphere, it contains less moisture, and is additionally dried upon delivery to the salon. This is done so that ice does not freeze in the pipes of the air conditioning system, which can lead to its blockage. That is why many passengers complain of dry eyes and throat during the flight.
RIA News
When using the information, a hyperlink to Eurasia Diary is required.
Oxygen
Almost all living organisms need oxygen. People breathe air, which is a mixture of gases, a large part of which is it.
The inhabitants of the aquatic environment also need this substance, so the concentration of oxygen in water is a very important indicator. Usually it is up to 14 mg / l, when it comes to natural waters, and sometimes even more. The same liquid that flows from the tap contains much less oxygen, and this is easy to explain. Tap water after water intake goes through several stages of purification, and dissolved oxygen is an extremely unstable compound. As a result of gas exchange with the air, most of it simply evaporates. So where does the oxygen in water come from, if not from the air?
In fact, this is not entirely true, it is also taken from the air, but its share, dissolved as a result of contact with the atmosphere, is extremely small. In order for the interaction of oxygen with water to be sufficiently effective, special conditions are necessary: low temperature, high pressure and relatively low salinity. They are far from always observed, and life would hardly exist in its present form if the only way for the formation of this gas in the aquatic environment was interaction with the atmosphere. Fortunately, there are two more sources where oxygen in water comes from. Firstly, dissolved gas molecules are found in large quantities in snow and rain waters, and secondly - and this is the main source - as a result of photosynthesis carried out by aquatic vegetation and phytoplankton.
By the way, despite the fact that the water molecule contains oxygen, living organisms, of course, are not able to extract it from there.Therefore, it remains for them to be content with the dissolved share.
Sources of gases dissolved in water
But where do all these substances come from in water? Nitrogen, as a rule, dissolves in the process of interaction with the atmosphere, methane - as a result of contact with rocks and decomposition of bottom silt, and hydrogen sulfide is formed as a product of decay of organic residues. As a rule, hydrogen sulfide is contained in deep water layers and does not rise to the surface. With its high concentration, life is impossible, for example, in the Black Sea at depths of more than 150-200 meters, due to the high saturation of water with hydrogen sulfide, there are almost no living organisms, except for some bacteria.
Oxygen is also always contained in water. It is a universal oxidizing agent, therefore it partially decomposes hydrogen sulfide, reducing its concentration. But where does the oxygen in water come from? There will be a special discussion about him.
where does the moisture in the atmosphere come from
In the air, these are microaerosols (MA), in water, they are microsuspensions (MV). Their property is that they remain insoluble in water or do not evaporate in the air, remaining in a solid state.
Due to their small size (from a few microns to tenths of a mm) in a moving medium (air, water), due to turbulent eddies, they practically do not settle under the action of gravity and are in a "suspended" state.
MA and MA can be both inorganic (microparticles of rocks, sand, etc.) and organic origin (microbes, bacteria, viruses, micromites, scales and villi of animal and plant integuments, etc.).
See Fig. i: Inorganic MA and MB can have both "terrestrial" and "cosmic" origin. As you know, the Earth, flying in orbit, "rakes" from space with its atmosphere (like a "vacuum cleaner") a lot of cosmic bodies of various sizes - from meteorites that reach the Earth and meteors (burning from friction against the atmosphere, they also give MA) to the smallest cosmic particles (cosmic dust), which gradually settle down, remaining in the atmosphere (MA) or falling into the water (MV); due to this, the mass of the Earth increases to 100 tons per day, see:
MA and MW of "terrestrial" origin are both particles of rocks, and crystals of salts, smoke, etc.
e., raised from the surface of the Earth (and the bottom of reservoirs) into air and water, respectively, by flows and turbulent eddies of air (MA) and water (MW) and remaining in the volume of water and air. At the same time, both in the lower layer of the atmosphere and in the water there are many MA and MA of purely organic origin.
It is important to note that counting with microscopes showed that the amount of MA and MB can be very large even if the air and water remain relatively transparent (up to 30 thousand
particles in each cube. cm of water or air), but if the amount of MA and MB becomes too large, then the phenomenon of "haze" occurs in the air, even with dry air (especially with smoke), and in water they speak of its "turbidity". An excess of MA and MA is harmful to human health, therefore, with an excess of MA, special protective masks (or even gas masks) are used to protect the respiratory organs, and with an excess of MA in water, it is specially filtered from mechanical suspensions using various filters before eating.
The cleanest from MA above the Earth is the air above Antarctica, see: But in nature, the role of MA and MW is quite large. In water, the presence of MW allows them to serve as “crystallization nuclei”, on which ice crystals begin to grow as the temperature decreases. In the air, MA is an important component of the atmosphere, since it is due to MA that water vapor condenses (fog, clouds) or sublimes (ice fog, high crystalline clouds) on them. Due to condensation and sublimation, clouds and precipitation arise, and since precipitation is the only source of water on land, without MA they would not have arisen and the entire land would have turned into a dead, lifeless desert,and life on our planet would remain only in water (oceans, seas). So thanks to MA for letting us live on land! And lastly, at altitudes of more than 8-10 km, there is very little MA, and even when the air is saturated with water vapor at low temperatures, it becomes "nothing to condense and sublimate", in connection with which high-altitude aircraft, throwing combustion products from engines, leave condensation follow the plane, for more details see:
Stones carried by water
Imagine a flowing river. Or the flow of water from an outlet. A slowly flowing river drags grains of sand with it. What weight stones
will be carried away by a river flowing twice as fast? And how will the fish react?
that you install a more powerful filter. Twice as heavy stones? Three times?
No. Twice as fast current of water carries stones with it
64 (sixty-four) times more severe. And the fish will not see such a current
sugar. In hydrology, this is called Erie's Law, which states that an increase in
flow rate n times informs the flow of the ability
drag objects with you to n6.
Why this is so can be illustrated by the example of a cube
with edge length a.
The force of the water flow F acts on the face of the cube,
which tends to rotate it around the edge passing through the point A
and perpendicular to the drawing plane. This is prevented by the weight of the cube in the water.
P. To keep the cube in balance, it is necessary
equality of moments about the axis of rotation. The equality of the moments gives:
F a/2 = P a/2 or F=P
The law of conservation of momentum gives:
ft=mv
where: t is the duration
the action of the force, m is the mass of water involved in
pressure in time t. The mass of water flowing
to the side face is equal to (the density of water is equal to unity, for simplicity we use the system
GHS):
m=a2vt
Hence, assuming the time equal to a second, we obtain from the condition
equilibrium rib size (w is the density of the material
Cuba):
a=v2/(w-1)
The edge of a cube that can resist the flow of water is proportional to
square of the flow rate. The weight of a cube is proportional to the volume of the cube, i.e. third degree
its linear dimensions. Hence the weight of the cube carried by the water is proportional to the sixth
the rate of water flow. And if a calm current can roll grains of sand
weighing half a gram, then a river twice as fast carries along pebbles weighing 32 grams,
and twice as fast mountain river - stones weighing about two kilograms. Remember about
this when you put in a powerful filter.
cavitation as the reason
Before you start clarifying the issue, it is important to know: pumps are installed depending on the diameter of the well! For sizes up to 100 mm a submersible pump is suitable, smaller diameters require a circular or plunger pump. What is cavitation? This is a violation of the continuity of the liquid flow, otherwise - filling the water with bubbles
Cavitation occurs in those areas where the pressure drop reaches a critical rate. The process is accompanied by the formation of voids in the flow, the release of bubble formations of air that appear due to vapors and gases released from the liquid. Being in the area of reduced pressure, the bubbles can grow and collect into large hollow caverns, which are carried away by the fluid flow and, in the presence of high pressure, collapse without a trace, and in the conditions of an ordinary domestic well, they often remain and it turns out that the pump during operation pumps air bubbles from wells without producing the required volume of water
What is cavitation? This is a violation of the continuity of the liquid flow, otherwise - filling the water with bubbles. Cavitation occurs in those areas where the pressure drop reaches a critical rate. The process is accompanied by the formation of voids in the flow, the release of bubble formations of air that appear due to vapors and gases released from the liquid.Being in the area of reduced pressure, the bubbles can grow and collect into large hollow caverns, which are carried away by the fluid flow and, in the presence of high pressure, collapse without a trace, and in the conditions of an ordinary domestic well, they often remain and it turns out that the pump during operation pumps air bubbles from wells without producing the required amount of water.
The identification of the cavitation zone is sometimes impossible due to the lack of special instruments, but it is important to know that such a zone can be unstable. If the disadvantage is not eliminated, then the consequences can be devastating: vibration, dynamic effects on the flow - all this leads to a breakdown of the pumps, because each device is characterized by a specified value of cavitation reserve
Otherwise, the pump has a minimum pressure, within which the water that has entered the device retains its density properties. With changes in pressure, caverns and air voids are inevitable. Therefore, the selection of the pump should be carried out depending on the volume of water needed to meet economic and domestic needs.
Physical characteristics of air
Transparency, lack of color and smell of the gaseous atmosphere that surrounds us, from their own life experience, are well known to students in grade 2. The properties of air, for example, its lightness and mobility, can be explained to the children using the example of wind farms. They are built on hills and hills. After all, the speed of air movement depends on the height. Such power plants are safe in operation and do not harm the environment.
Like other substances, the components of the atmosphere have mass. To solve problems in the course of inorganic chemistry, it is generally accepted that the relative molecular weight of air is 29. Given this value, you can find out which gases are lighter than the atmosphere.
These include, for example, helium, hydrogen. To create an aircraft, a person conducted experiments and studied the properties of air. The experiments were crowned with success, and the first flight in the world was carried out by the French inventors, the Montgolfier brothers, already in the 18th century. The shell of their balloon was filled with a hot mixture of hydrogen, nitrogen and oxygen.
Airships - more maneuverable and better controlled devices, rise up because their shells are filled with light gases, namely helium or hydrogen. Man uses the ability of a gas mixture to compress in devices such as air brakes. They are equipped with buses, subway trains, trolleybuses. The examples given are a clear illustration of how a person uses the properties of air.
RK in artificially created ecosystems
Good aeration is essential, for example, in the aquarium hobby. That is why it is necessary not only to install special pumps that pump air into the water and saturate it with oxygen, but also, for example, if necessary, plant various algae at the bottom
Of course, those who have such a hobby are primarily interested in the aesthetics of the ecosystem, but we must not forget about its stability and some kind of durability.
If we are talking about fisheries, pearl production and other specific industries of this type, then in addition to various measures aimed at maintaining a sufficient concentration of dissolved oxygen in the water, it is necessary to regularly measure this indicator using special samples.
When taking them, it is extremely important that there is no contact with air, this can distort the results of the analysis.
Fish, mollusks and other inhabitants of the seas and oceans have always fascinated people with their measured pace of life, graceful movements of their bodies. The inhabitants of the water world amaze with the variety of their shapes and colors. Despite the cardinal differences with mammals, an indispensable condition for their existence is the presence of oxygen in the water.
Where does the oxygen in water come from?
Water, like air, is oxygenated by plants.At the same time, only 20 percent of oxygen supply depends on its release by terrestrial plants - mainly tropical forests, and 80 percent - by ocean and seaweeds - phytoplankton. Therefore, the ocean is rightly called the lungs of the planet Earth. In the cells of blue-green algae, which form the basis of phytoplankton, a photosynthesis reaction occurs, as a result of which a mixture of carbon dioxide and water is converted into glucose.
As a result, oxygen is released in large quantities. The energy needed for photosynthesis is provided by sunlight. Glucose is a source of nutrition for plants, and oxygen is necessary for respiration.
How do fish get oxygen dissolved in water?
Fish breathe through gills. They are located in paired openings - gill slits, and are pierced by numerous blood vessels. This organ was formed as a result of a long process of evolution due to the protrusion of the walls of the pharynx and the outer cover. This is a kind of pump, the work of which is provided by the skeleton of the fish and the muscles of the gill arches, which alternately close and open the gill covers. Through the mouth, water enters the gills, gives the capillaries of the blood vessels the oxygen dissolved in the water, and is pushed back.
What is used in home aquariums to saturate the water with oxygen
To increase the degree of oxygenation of water in aquariums, both special equipment and preparations are used to enhance the growth of aquarium plants.
The simplest of the methods of enrichment with oxygen is aeration - blowing air through the water column. This method allows you to equalize the temperature of the water in the aquarium by mixing the layers of water, increases the permeability of the soil. These actions eliminate such troubles as the decay of organic residues and the release of ammonia, methane and hydrogen sulfide. Aeration of water is carried out using an aquarium compressor, which pumps air to the bottom of the aquarium, and then, in the form of bubbles, the air rises through the water column. In this case, the water is saturated with oxygen, which is necessary for the respiration of plants and fish.
It will also be useful to use special biological preparations for the daily care of aquatic plants. Indeed, in addition to oxygen, the underwater garden releases a large number of enzymes and vitamins necessary for fish, and prevents the reproduction of pathogenic microbes in the aquarium.
Composition and properties of air
An example illustrating the fact of the ability of the elements of the atmosphere to absorb thermal energy, to put it more simply, to heat up, will be as follows: if the gas outlet tube of a preheated flask with a ground stopper is lowered into a container with cold water, then air bubbles will come out of the tube. The heated mixture of nitrogen and oxygen expands, no longer fitting into the container. Part of the air is released and enters the water. When the flask is cooled, the volume of gas in it decreases and contracts, and water flows up the flask through the gas outlet tube.
Consider another experiment conducted in the lessons of natural history for students in grade 2
The properties of air, such as elasticity and pressure, are clearly visible if an inflated balloon is squeezed with the palms of your hands, and then carefully pierced with a needle. A sharp pop and flying flaps demonstrate gas pressure to children
It can also be explained to students that man has applied these properties in the manufacture of pneumatic devices, such as jackhammers, pumps for inflating bicycle tubes, pneumatic weapons.
Water from the tap comes in jerky jerks with air why
Water from the tap comes in jerks (jolts) with air - why?
This happens after the water is turned off and the water pipes (networks) are repaired.
Air got into the system, water comes in jerks, jerks, the same air comes out with a hiss.
The easiest, but not the most correct option for a particular user, is to remove the aerator
When the pressure is working, the air will leave the system, hissing and jerking will stop.
And not the right option, because the user “drives” through his water meters, through the filter, and if he has fine filters installed, then after such a “run” of rusty water, cartridges and filter fillers will have to be changed.
Do nothing, wait until the neighbors in the riser above and below drive rusty water through their taps and faucets, counters, filters.
And you just have to unscrew the coarse filter mesh, rinse it, put it in place and that's it.
Well, or take a “hit” on yourself, drive all this dirt through your pipes, filters, taps.
If after the root taps (on the DHW and cold water risers) “Americans” are installed,
If the Americans are right after the riser (sometimes this happens), before the main taps, then of course this option is not working.
In fact, you gave the answer in your question. The water from the tap comes with air as the system is airy. Most likely, repair work was carried out on the pipeline, as a result of which air got into the system. When water is supplied to the system, the water pushes this air out and it turns out that the water from the tap, as it were, comes in jolts.
This often happens after stopping the water supply to the system and completely or partially draining it. After the supply is resumed, the air does not immediately leave the system - it is blown away by water pressure.
When we turn on the faucet, we release air, which comes out much faster than water. Its place in the pipes is filled with water and it partially comes out mixed with air. The air in the system is not evenly distributed, often leaving “plugs” in the upper levels. It is these air “plugs” that begin to spit when the tap is opened, then with air, then with water. So that after stopping the water this does not happen, just open the tap a little to bleed the air. The water ran steadily - you can use it.
When repairing a water supply or sewage system, the water supply to the riser or the weight of the house is blocked. Then the remaining water in the pipes is drained so that it does not interfere with the repair. Instead of water, the pipes are filled with air spontaneously. After the malfunction is eliminated, water is turned on, it begins to fill the pipes. When filling the pipes with water, the air is compressed to the same pressure as the pressure becomes in the pipes when water is supplied. When the faucet is opened, air under pressure comes out of it, then air goes mixed with water, and only then water begins to flow. True, at first the water is dirty. After a while the water becomes clear.
This happens because the water is supplied according to the schedule and during the time when it is not pumped, air is sucked into the system, and after the pumps are turned on, this air mixed with water literally shoots from the tap through the pipes, it can damage both the taps and the washing machine, for example, break the gears water meter, tear off the supply hoses from the toilet bowl or faucets.
therefore, it is strictly forbidden to open blue in this case, as well as turn on gas water heaters, washing machines, it is advisable to block the supply to the toilet, so as not to damage something there.
Therefore, this phenomenon is not only incredibly annoying, but also fraught with serious equipment breakdowns.
What to do in such cases, the best option is to close the common valve at the inlet and wait until the pressure in the system rises to a level where the air is evenly mixed with water and it will flow at least more or less stably, in this case the water flows with a hiss and white filled with air bubbles.
So there is only one way out, to wait and be patient, sometimes you can never wait for water, but turn on the water when your gas column flies off the hinges and like a bullet the strainer flies off the aerator, I think it’s very uncomfortable.
It is necessary to quarrel with the water supplier, let them at least solve the problem by reducing the payment for bleeding air, draw up acts and write off the cubic capacity necessary to bleed air from the system in areas where there is such a problem.
a source
Air impurities Microbes, Dust, Viruses.
The main constituents of air are oxygen and nitrogen; as we have already mentioned, oxygen makes up about one-fifth of the air, and nitrogen about four-fifths. But there are other substances in the composition of the air.
Air always contains some moisture in the form of water vapor; so, for example, a room with an area of 10 square meters can contain about 1 kilogram of water vapor, invisible to the eye; this means that if all the steam contained in the room is collected and turned into water, then 1 liter of water will be obtained. If in winter, for example, you enter a warm room from the cold, then the glasses are immediately covered with small water droplets (condensate); the reason for this is the water vapor in the air, which, like dew, settled on the glasses of the glasses. In summer, the amount of steam in a cubic meter of air can be 10 times greater than in winter.
In addition, an insignificant amount of carbon dioxide enters the air (namely, 3 parts of carbon dioxide account for 10,000 parts of air); however, this gas plays a very important role in the natural balance. The human body produces a large amount of carbon dioxide and releases it from itself during the exhalation of air. The air exhaled by a person contains more than 4 percent carbon dioxide. This air is no longer breathable. In general, air that contains more than 5 percent carbon dioxide acts on a person in a toxic way; a person cannot stay in such air for a long time - death will come.
Also, the air, especially in large cities, is infected with various bacteria, they are often called microbes, and viruses. These are the smallest invisible living beings; they can only be seen with a microscope magnifying a hundred or a thousand times. In a favorable environment, they multiply extremely quickly and this reproduction is very simple. A living microbe narrows in the middle of its body and finally divides in half; thus, by simple division from one microbe, two are obtained. Due to the ability to multiply so quickly, bacteria and viruses are the main enemy of mankind. Many of our illnesses, from colds and flu to AIDS, come from viruses and microbes. These creatures are carried in huge numbers in the air and are carried by the wind in all directions, they are both in the water and in the earth. We inhale or swallow them in hundreds and thousands, and if they find in a person a fertile ground for their reproduction, then the disease is ready: there is fever, weakness, and various unpleasant symptoms. Sometimes these bacteria and viruses imperceptibly, slowly, without even causing much pain, but systematically undermine health and destroy the body, leading to death, as in tuberculosis or AIDS.
In room dust, bacteria find favorable soil for their reproduction. This dust always rises from the floor and fills the rooms. Usually we do not see this dust; but sometimes in the summer, when the sun's rays enter the window, it is easy to notice in the sun's rays how millions of dust particles rush in the air. Where does room dust come from? We bring it with us from the street on our feet, dust enters through windows and doors; in addition, the smallest particles come off the floor and from various objects. This dust we inhale; it rests on our lungs; weakens our health and imperceptibly shortens our lives.
Dust in the atmosphere has a variety of origins; dust is lifted from the ground by the wind; smoke from chimneys, products of eruptions from volcanoes, and so on, all this is mixed by the wind and carried hundreds, sometimes thousands of kilometers across the earth's surface.
In places covered with forests, the air is cleaner, because the forest cleans the air with its leaves as a filter, and, in addition, the forest traps the wind that spreads dust.In the upper layers of the atmosphere, the air is cleaner, since less earth dust is brought there by the wind. In mountainous areas, the air is also much healthier. Therefore, sanatoriums for the sick are arranged mainly on an elevated, wooded area. Near the seas, the air is also distinguished by purity and high humidity, and is useful for patients, for example, with asthma.
Elimination of cavitation
What can be done to avoid the appearance of air in the well and the entry of water with bubbles:
- Replacing the suction pipe of small diameter with a larger one;
- Moving the pump closer to the storage tank.
- Reduce the pressure of the suction element by replacing it with a smooth pipe, and the valve can be replaced with a gate valve, and the check valve can be removed altogether;
- The presence of a large number of turns in the suction pipe is unacceptable, they must be reduced or the bends of a small radius of turns should be replaced with large ones. The easiest way is to align all the bends in the same plane, and sometimes it is easier to replace rigid pipes with flexible ones.
If all else fails, you will have to increase the pressure on the suction side of the pump by raising the level of the tank, lowering the axis of the pump installation or connecting a booster pump.
About plugs and small bubbles
It is clear that air can occupy the entire pipe along some of its length. This is an airlock. It is insurmountable for natural circulation and for small (conventional) circulation pumps. But there may be small bubbles that rush through the system along with the water. Such bubbles can simply circulate, or they can unite when they meet. If there is a place in the system to collect these bubbles, then during the operation of the heating system, an air plug will collect in this place. After that, the circulation will stop. Bubbles can also collect in traps (radiators). In this case, the part of the radiator in which air has collected becomes cold.
If the circulation in our system is quite fast, and there are no obvious humps and traps, then bubbles circulate through the system and create gurgling sounds. As if water is pouring in a thin stream from one container to another. I regularly hear this kind of noise in one of my bathrooms, which has a beautiful, but not very well-configured heated towel rail. Bubbles run through it so actively that some parts of the heated towel rail I have are either cold or hot.
Danger of air bubbles in the pipeline
Bubbles, especially large ones, can destroy even strong line elements. The main troubles that they cause to owners of private houses:
- They accumulate in the same areas, leading to breakage of pipe sections and adapters. They also pose a hazard to curved and winding pipe sections where air is trapped.
- They break the water flow, which is inconvenient for the user. Faucets all the time "spit out" water, vibrate.
- Provoke hydraulic shock.
Water hammer leads to the formation of longitudinal cracks, due to which the pipes are gradually destroyed. As time passes, the pipe breaks at the place of cracking, and the system ceases to function.
Therefore, it is important to equip additional elements that allow you to quickly get rid of dangerous bubbles.