4 Methods of heat transfer in heat exchange equipment
Heat transfer -
complex process that, when studied
divided into simple phenomena. Distinguish
three elementary methods of transfer
heat: conduction, convection
and thermal radiation.
1) Thermal conductivity
- heat transfer process
through direct contact
microparticles having different
temperature, or contact of bodies
(or parts thereof) when the body does not move
in space. Thermal Conduction Process
associated with temperature distribution
inside the body. Temperature characterizes
degree of heating and thermal state
body. Set of temperature values
at various points in space
different points in time is called
temperature
field
(stationary or non-stationary).
Isothermal
surface
is the locus of points of the same
temperature. Any isothermal
the surface divides the body into two
areas: with higher and lower temperatures;
heat passes through an isothermal
surface to lower
temperature. The amount of heat ΔQ,
J passing per unit time Δτ,
s, through an arbitrary isothermal
surface is called thermal
flow Q,
Tue
Characteristic
heat flow - density
heat flow
(specific heat flux).
Mathematical
expression of the law of heat conduction
Fourier:
 .
.
Multiplier λ -
coefficient
thermal conductivity,
W / (m K), numerically equal to the number
heat passing per unit of time,
through a unit of surface, with a difference
temperatures per degree, per unit
one meter long.
2) Convection
– movement of macroscopic parts
environment (gas, liquid), leading to
transfer of mass and heat. per process
heat transfer by convection is affected by:
1. The nature of the movement
liquid near a solid wall (free
or forced - laminar or
turbulent). Fluid flow mode
determined not only by speed, but also
dimensionless complex number
Reynolds Re
= ωlυ.
2. Physical
properties or type of liquid. For heat dissipation
density, heat capacity,
thermal conductivity coefficients and
thermal diffusivity, kinematic
the viscosity of the liquid.
3. Thermal conditions
mode (for example, changing the aggregate
states).
4. Temperature
pressure ΔT
is the temperature difference between the solid
wall and liquid.
5. Direction
heat flow Q
(heat transfer from hot to cold wall)
more liquid).
6. Geometric
body dimensions that affect thickness
boundary layer.
7. Direction
heat transfer surface.
convective process
heat transfer is described by Newton's law
 ,
,
W,
where α is the coefficient
heat transfer, W/(m2 K),
numerically equal to the amount of heat,
transferred from liquid to solid
surface per unit time, through
unit of surface at drop
temperature between wall and liquid
one degree.
3) All bodies are continuous
sent to their surroundings
electromagnetic waves of various lengths.
Wave radiation is always transforming
into thermal energy. For light and
infrared rays (0.4 ... 800 microns) is
the transformation is most pronounced
and these rays are called thermal, and
the process of their distribution thermal
radiation
or radiation.
Thermal radiation intensity
increases sharply with increasing temperature.
falling on the body
The radiant stream consists of three parts:
reflected, absorbed and transmitted.
reflective
ability
R
is the ratio of reflected energy to
energy falling on the body (total).
absorbent
ability
A
is the ratio of absorbed energy to
energy falling on the body (total).
throughput
ability
D
is the ratio of energy passing through
body, to the energy falling on the body (total).
In accordance with
energy conservation law: R
+ A
+ D
= 1.
Total
heat transfer by radiation (law
radiant heat transfer), W,
 ,
,
where εP
is the reduced emissivity of the system
bodies; WithO=5,67
W/(m2 K4)
– emissivity is absolutely
black body; F
is the area of the heat transfer surface,
m2.
These processes
occur at the same time, influence each other
friend - difficult
heat exchange.
In real conditions, convection is always
accompanied by heat conduction or
molecular heat transfer.
Joint heat transfer process
convection and heat conduction
called convective
heat exchange.
Convective heat transfer between liquid
and a solid body is called heat dissipation.
The transfer of heat from a hot liquid to
cold through the wall separating them
– heat transfer.
Pressure
Pressure
–
it
force impact (F)
the body and its parts to the environment
or shell and on adjacent parts of that
the same body per unit area (S).
This force is directed
perpendicular to any element
surface and balanced back
directional force
environment, shell or adjacent
element of the same body.
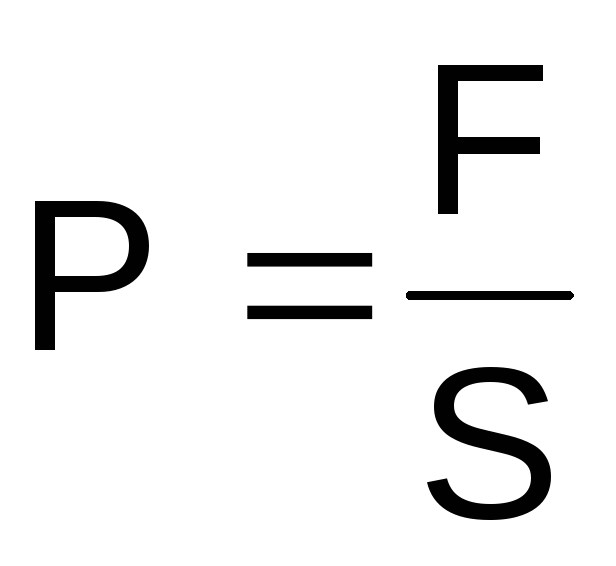
V
The SI unit of pressure is the pascal
(Pa) is 1 N/m2,
those. force of one newton acting on
normals to an area of one square
meter. For technical measurements Pascal
very small value, so we introduced
Pascal multiple unit of pressure bar:
1 bar = 105
Pa. Selecting this pressure unit
is explained by the fact that atmospheric
air pressure above the earth's surface
approximately equal to one bar.
V
technique is often used unit
pressure in the old measuring system
(GHS) - technical
atmosphere:
1 atm = 1 kgf/cm2
(not to be confused with the concept of physical
atmosphere).
Often
measure pressure, especially small,
liquid column height (mercury, water,
alcohol, etc.). Liquid column (Fig. 1.5)
produces pressure on the base of the vessel,
defined by equality
R
= F/S = HSρg/S
= ρgH,
(1.4)
where
ρ is the liquid density, kg/m3;
H
is the height of the liquid column, m;
g
– free fall acceleration, m/s2;
F,
S is the force acting on the bottom of the vessel, and
its area.
From
equation (1.4) it follows that the pressure Р
corresponds to the height of the liquid column
H = P/(ρg), i.e. height H is directly proportional
pressure, since ρg is the quantity
constant.
V
practice the height of the liquid column often
taken to assess pressure. Therefore meters
and millimeters of liquid steel column
pressure units. For
transition from the height of the liquid column to
pascals are needed in formula (1.4)
substitute all quantities in SI.
For instance,
at 0°C
water density is 1000 kg/m3,
mercury – 13595 kg/m3
in earth conditions. Substituting these quantities
into formula (1.4), we obtain relations for
1mm column of these liquids and pressure in
pascals:
H
= 1 mm water column corresponds to Р= 103 9.81 10-3=
9.81 Pa;
H
= 1 mmHg corresponds to Р = 13595 9.81 10-3=
133.37 Pa.
At
determination of pressure by column height
fluid must take into account the change
its density as a function of temperature.
This must be done to match
pressure measurement results. So,
when determining atmospheric pressure
using a mercury barometer
readings are reduced to 0 °C
based on the ratio
VO
\u003d B (1 - 0.000172 t),
(1.5)
where
B is the actual height of the mercury
barometer column at mercury temperature
tоС;
VO
- barometer readings reduced to
temperature 0 °C.
V
calculations use column pressures
liquids brought to temperature 0
OS.
Measurement
pressure
in technology based on indications
various devices operating on
the principle of reflection on the magnitude scale,
numerically equal to the pressure difference in
measuring point and ambient pressure
environment. Typically, devices are
positive scale, i.e. difference between
more and less pressure. So
they are divided into devices for measuring pressure:
more
atmospheric –pressure gauges,
less than atmospheric –vacuum gauges.
P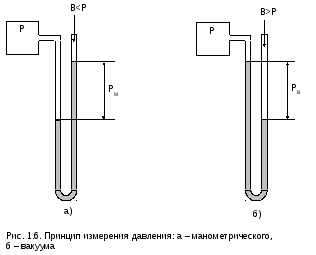
such devices in the form of liquid
U-shaped pressure gauges (vacuum gauges)
shown in fig. 1.6.
Pressure
on the scale of these instruments is called
gauge pressure PM
and vacuum RV
respectively. Pressure at the measuring point
is called absolute P, surrounding
environment - pressure of atmospheric air
or barometric B, since the instrument,
usually installed in the surrounding
its atmospheric air.
Estimated
instrument pressure dependences will be
the following:
manometric
pressure:
RM
\u003d P - B,
(1.6)
where
RM
- gauge pressure (according to the instrument);
R
– absolute pressure;
V
– atmospheric air pressure
(barometric pressure);
vacuum:
RV
\u003d B - P,
(1.7)
where
RV
- vacuum (vacuum gauge readings).
Parameter
states of a thermodynamic body
is the absolute pressure, at
using appliances, it will
determined according to the type
device according to the following dependencies:
for
manometer
R
= PM
+ V,
(1.8)
for
vacuum gauge
R
= B - PV
. (1.9)
Coordination of water temperature in the boiler and system
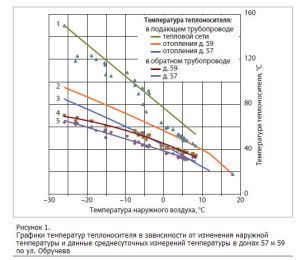
There are two options for coordinating high-temperature coolants in the boiler and lower temperatures in the heating system:
- In the first case, the efficiency of the boiler should be neglected and, at the exit from it, the coolant should be given out to such a degree of heating that the system currently requires. This is how small boilers operate. But in the end, it turns out not always to supply the coolant in accordance with the optimal temperature regime according to the schedule (read: “Heating season schedule - beginning and end of the season“). Recently, more and more often, in small boiler rooms, a water heating regulator is mounted at the outlet, taking into account the readings, which fixes the coolant temperature sensor.
- In the second case, the heating of water for transportation through networks at the outlet of the boiler room is maximized. Further, in the immediate vicinity of the consumers, the temperature of the heat carrier is automatically controlled to the required values. This method is considered more progressive, it is used in many large heating networks, and since regulators and sensors have become cheaper, it is increasingly used in small heat supply facilities.
Ways to reduce heat loss
But it is important to remember that the temperature in the room is affected not only by the temperature of the coolant, outdoor air and wind strength. The degree of insulation of the facade, doors and windows in the house should also be taken into account.
To reduce the heat loss of housing, you need to worry about its maximum thermal insulation. Insulated walls, sealed doors, metal-plastic windows will help reduce heat leakage. It will also reduce heating costs.
(No ratings yet)
The concept of the heating rate can be completely different for two situations: when the apartment is heated centrally, and when autonomous heating is installed and functioning in the house.
Centralized heating in the apartment
Optimal values in an individual heating system

It is important to ensure that the heat carrier in the network does not cool below 70 ° C. 80 °C is considered optimal
It is easier to control heating with a gas boiler, because manufacturers limit the possibility of heating the coolant to 90 ° C. Using sensors to adjust the gas supply, the heating of the coolant can be controlled.
A little more difficult with solid fuel devices, they do not regulate the heating of the liquid, and can easily turn it into steam. And it is impossible to reduce the heat from coal or wood by turning the knob in such a situation.At the same time, the control of heating of the coolant is rather conditional with high errors and is performed by rotary thermostats and mechanical dampers.
Electric boilers allow you to smoothly adjust the heating of the coolant from 30 to 90 ° C. They are equipped with an excellent overheating protection system.
Advantages of using the regulator in heat supply
The use of the regulator in the heating system has the following positive aspects:
- it allows you to clearly maintain the temperature schedule, which is based on the calculation of the temperature of the coolant (read: “Correct calculation of the coolant in the heating system“);
- increased heating of water in the system is not allowed and thus economical consumption of fuel and thermal energy is ensured;
- heat production and its transportation take place in boiler houses with the most efficient parameters, and the necessary characteristics of the coolant and hot water for heating are created by the regulator in the heating unit or point closest to the consumer (read: "Heat carrier for the heating system - pressure and speed parameters");
- for all subscribers of the heating network, the same conditions are provided, regardless of the distance to the source of heat supply.
Specific volume
Specific
volume
– it
volume per unit mass of a substance (m3/kg):
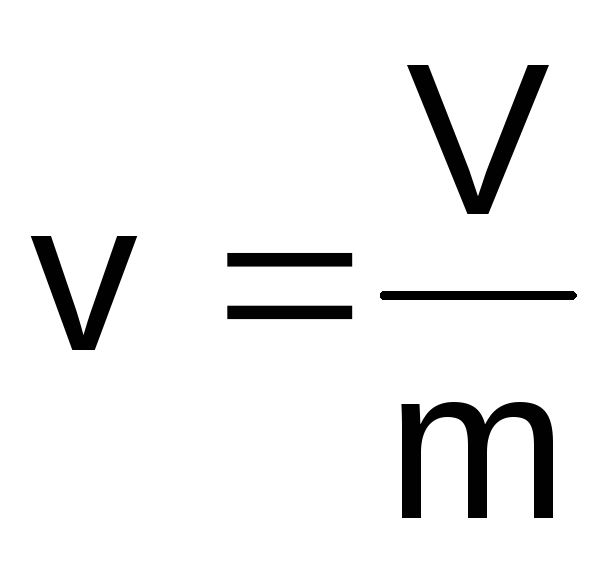
(1.1)
where
V is the volume of the body, m3;
m - body weight, kg.
value,
reciprocal of specific volume is called
density
(kg/m3):
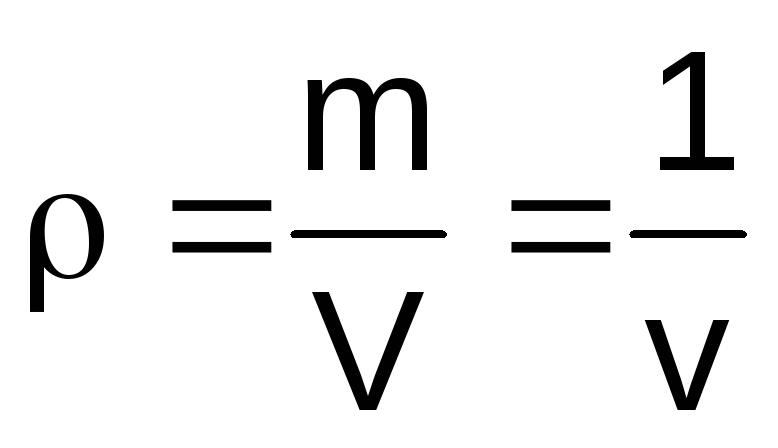
(1.2)
V
practice is often used concept
specific gravity
is the weight per unit volume of the body (N/m3):
 ,
,
(1.3)
where
g
–
acceleration of gravity
(approximately 9.81 m/s2).
At
converting any value to SI, for example
from 1 g/cm3,
should be guided by the following
rule: all quantities of formula (1.3)
represent in SI units and perform
with them operations arithmetic
formula operators:
=
1 g/cm3
= 9,81·10-3/10-6
= 9,81·103
N/m3.
At
it must be remembered that 1 kgf \u003d 9.81 N. This
ratio is often used for
conversion of non-system units to SI.
Calculation of the temperature regime of heating
When calculating the heat supply, the properties of all components must be taken into account. This is especially true for radiators. What is the optimal temperature in the radiators - + 70 ° C or + 95 ° C? It all depends on the thermal calculation, which is performed at the design stage.
An example of drawing up a heating temperature schedule
First you need to determine the heat loss in the building. Based on the data obtained, a boiler with the appropriate power is selected. Then comes the most difficult design stage - determining the parameters of heat supply batteries.
They must have a certain level of heat transfer, which will affect the temperature curve of the water in the heating system. Manufacturers indicate this parameter, but only for a certain mode of operation of the system.
If you need to spend 2 kW of thermal energy to maintain a comfortable level of air heating in a room, then the radiators must have no less heat transfer.
To determine this, you need to know the following quantities:
- The maximum water temperature in the heating system is allowed -t1. It depends on the power of the boiler, the temperature limit of exposure to pipes (especially polymer pipes);
- The optimum temperature that should be in the heating return pipes is t This is determined by the type of mains wiring (one-pipe or two-pipe) and the total length of the system;
- Required degree of air heating in the room –t.
With this data, you can calculate the temperature difference of the battery using the following formula:
Next, to determine the power of the radiator, you should use the following formula:
Where k is the heat transfer coefficient of the heating device. This parameter must be specified in the passport; F is the radiator area; Tnap - thermal pressure.
By varying various indicators of the maximum and minimum water temperatures in the heating system, you can determine the optimal mode of operation of the system
It is important to correctly initially calculate the required power of the heater. Most often, the indicator of low temperature in heating batteries is associated with heating design errors.
Experts recommend adding a small margin to the obtained value of the radiator power - about 5%. This will be needed in case of a critical decrease in the temperature outside in the winter.
Most manufacturers indicate the heat output of radiators according to the accepted standards EN 442 for mode 75/65/20. This corresponds to the norm of the heating temperature in the apartment.
1. Description of the design object and selection of heat supply systems
TO
protected ground structures
(cultivation facilities) include
greenhouses, greenhouses and insulated soil.
Widespread
greenhouses; they are classified according to
translucent fencing (glazed
and film) and by design (hangar
single-span and block
multi-span). Greenhouses operated
all year round, commonly called winter,
and used in spring, summer and autumn
- spring.
Heating
and ventilation of cultivation facilities
must support the given parameters
– temperature, relative humidity
and gas composition of the internal air,
as well as the required soil temperature.
Energy supply
greenhouses and greenhouses should be carried out
from district heating systems,
also allowed to use
gaseous fuel, electric
energy, geothermal waters and secondary
energy resources of industrial enterprises.
In winter greenhouses
it is necessary to provide water systems
heating the tent and soil, as well as
combined systems (water and
air).
Expediency
application of gas heating greenhouses
directly by combustion products
gaseous fuel or air
soil heating must be confirmed
technical and economic calculations.
At
water heating device
tent systems are recommended,
basement, soil and aboveground
heating. Coolant temperatures
(hot and reverse) for marquee,
ground and ground heating:
t
r =
150, 130 and 95 С,
t
O
= 70 С;
for soil heating: t
G
= 45 С
and t
O
= 30 С.
Water heating devices are necessary
place: in the upper zone - under the coating,
gutter trays and cornices (Fig.
5.1), in the middle zone - at the outer walls and
on the inner pillars of the cornice, at the bottom
zone - along the contour of the outer walls on
depth of 0.05 ... 0.1 m and for heating the soil -
at a depth of at least 0.4 m from the design
soil surface marks to the top of the pipes
heating.
Used for ground heating
asbestos cement or plastic
polyethylene and polypropylene
pipes. At coolant temperature
up to 40 ºС possible
use polyethylene pipes
temperature up to 60ºСpolypropylene pipes.
Usually they are attached to the opposite
collector of tent heating systems
with vertical steel bars.
Pipes must be laid evenly
by area of greenhouses at a distance,
determined by heat engineering
calculations. Application of steel pipes
for these purposes is not permitted.
Distance
between soil heating pipes
it is recommended to take equal to 0.4 m in
seedling department; 0.8 m and 1.6 m -
in other parts of the greenhouse.
With the air heating method, the air
with a temperature not exceeding 45 С
served in the working area of the greenhouse
perforated polyethylene
air ducts. These ducts must
be designed to provide uniform
supply of air and heat along the entire length.
In this section of the course project are given
detailed description of the design object
and selected heating systems,
layout of heating devices
all heating systems.
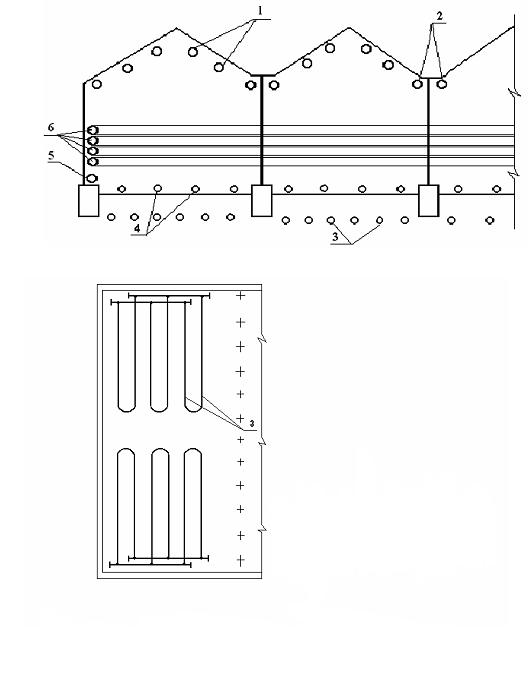
Rice.
5.1. A variant of the layout of heating
devices in a block-modular greenhouse
1
roof heating; 2 -
under tray heating; 3 -
soil heating; 4 -
ground heating; 5 -
basement heating; 6 - end (contour)
heating
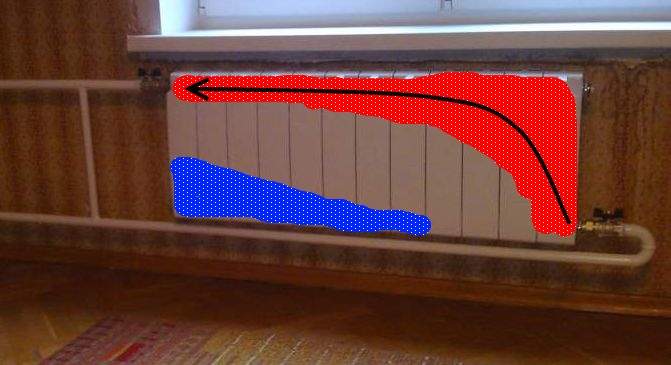
Single pipe heating system
Single-pipe heat supply of an apartment building has a lot of disadvantages, the main among which are significant heat losses in the process of transporting hot water. In this circuit, the coolant is supplied from the bottom up, after which it enters the batteries, gives off heat and returns back to the same pipe. For end consumers living on the upper floors, previously hot water reaches a barely warm state.
Another disadvantage of such heat supply is the impossibility of replacing the radiator during the heating season without draining the water from the entire system. In such cases, it is necessary to install jumpers, which makes it possible to turn off the battery and direct the coolant through them.
Thus, on the one hand, as a result of installing a single-pipe heating system circuit, savings are obtained, and on the other hand, serious problems arise regarding the distribution of heat among apartments. In them, the tenants freeze in the winter.
Heat carriers and their parameters
Estimated thermal power during the heating season, duration D zo.c, must be used partly at the current outside temperature tn.i and only when tn.r - fully.
Requirements for heating systems:
- sanitary and hygienic: maintaining the specified temperature of the air and the internal surfaces of the fences of the premises in time with allowable air mobility; limiting the surface temperature of heating devices;
— economic: minimal capital investments, economical consumption of thermal energy during operation;
- architectural and construction: compactness; linkage with building structures;
- production and installation: the minimum number of unified units and parts; mechanization of their production; reduction of manual labor during installation;
- operational: the effectiveness of the action during the entire period of work; durability, maintainability, non-failure operation; safety and quiet operation.
The most important are sanitary-hygienic and operational requirements, which determine the maintenance of a given temperature in the premises during the heating season.
Rice. 1.1. Changes in the average daily outdoor temperature during the year in Moscow:
tp - room temperature; tn1 - minimum average daily outdoor temperature
Classification of heating systems
Heating systems are divided into local and central.
V local systems for heating, as a rule, one room, all three elements are structurally combined in one installation, directly in which heat is received, transferred and transferred to the room. An example of a local heating system is heating stoves, the design and calculation of which will be discussed below, as well as heating systems using electrical energy.
Central are called systems intended for heating a group of premises from a single thermal center. Boilers or heat exchangers can be placed directly in the heated building (boiler room or local heating point) or outside the building - in the central heating point (CHP), at a thermal station (separate boiler house) or CHP.
The heat pipelines of the central systems are divided into mains (supply lines, through which the coolant is supplied, and return lines, through which the cooled coolant is discharged), risers (vertical pipes) and branches (horizontal pipes) connecting the lines with connections to heating devices.
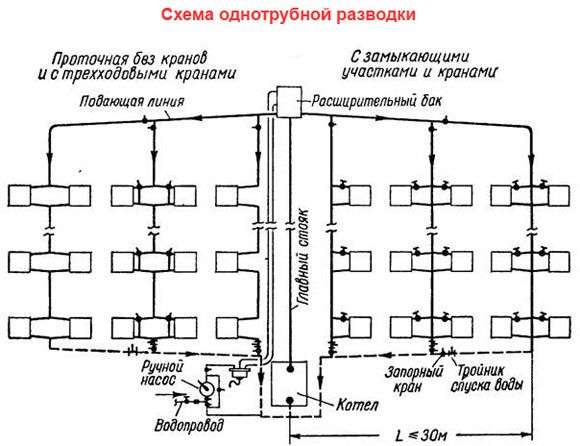
The central heating system is called regionalwhen a group of buildings is heated from a separate central heating plant. The coolant (usually water) is heated at a thermal station, moves along the outer (t1) and internal (inside the building tg t1) heat pipelines to the premises to the heating devices and, having cooled down, returns to the thermal station (Fig. 1.2).
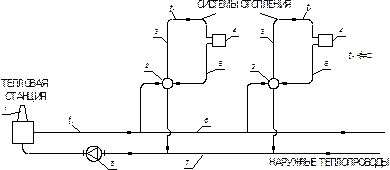 |
Rice. 1.2. Scheme of the district heating system:
1 – thermal station; 2 – local heating point; 3 and 5 – supply and return risers of the heating system; 4 - heating devices; 6 and 7 – external supply and return heat pipelines; 8 – circulation pump of the external heat pipe
As a rule, two coolants are used. The primary high-temperature heat carrier from the thermal plant moves through the city heat distribution pipelines to the central heating point or local heat points of buildings and back. The secondary heat carrier, after being heated in heat exchangers or mixed with the primary one, flows through the internal heat pipes to the heating devices of the heated premises and returns to the central heating station or the local heating point.
The primary coolant is usually water, less often steam or gaseous products of fuel combustion. If, for example, primary high-temperature water heats secondary water, then such a central heating system is called water-based. Similarly, there may be water-air, steam-water, gas-air and other central heating systems.
By type of secondary coolant, local and central heating systems are called water, steam, air or gas heating systems.
Date added: 2016-01-07; views: 1155;
Matching the temperature of the heat carrier and the boiler

The return temperature depends on the amount of liquid passing through it. The regulators cover the liquid supply and increase the difference between the return and supply to the level that is needed, and the necessary pointers are installed on the sensor.
If it is necessary to increase the flow, then a boost pump can be added to the network, which is controlled by a regulator. To reduce the heating of the supply, a “cold start” is used: that part of the liquid that has passed through the network is again transferred from the return to the inlet.
The regulator redistributes the supply and return flows according to the data taken by the sensor, and ensures strict temperature standards for the heating network.
How to raise the pressure
Pressure checks in the heating lines of multi-storey buildings are a must. They allow you to analyze the functionality of the system. A drop in pressure level, even by a small amount, can cause serious failures.
In the presence of centralized heating, the system is most often tested with cold water. The pressure drop for 0.5 hours by more than 0.06 MPa indicates the presence of a gust. If this is not observed, then the system is ready for operation.
Immediately before the start of the heating season, a test is performed with hot water supplied under maximum pressure.
Changes occurring in the heating system of a multi-storey building, most often do not depend on the owner of the apartment. Trying to influence the pressure is a pointless undertaking. The only thing that can be done is to eliminate air pockets that have appeared due to loose connections or improper adjustment of the air release valve.
A characteristic noise in the system indicates the presence of a problem. For heating appliances and pipes, this phenomenon is very dangerous:
- Loosening of threads and destruction of welded joints during vibration of the pipeline.
- Termination of the supply of coolant to individual risers or batteries due to difficulties in de-airing the system, the inability to adjust, which can lead to its defrosting.
- A decrease in the efficiency of the system if the coolant does not stop moving completely.
To prevent air from entering the system, it is necessary to inspect all connections and taps for water leakage before testing it in preparation for the heating season. If you hear a characteristic hiss during a test run of the system, immediately look for a leak and fix it.
You can apply a soapy solution to the joints and bubbles will appear where the tightness is broken.
Sometimes the pressure drops even after replacing old batteries with new aluminum ones. A thin film appears on the surface of this metal from contact with water. Hydrogen is a by-product of the reaction, and by compressing it, the pressure is reduced.
In this case, it is not worth interfering with the operation of the system - the problem is temporary and eventually goes away on its own. This happens only in the first time after the installation of radiators.
You can increase the pressure on the upper floors of a high-rise building by installing a circulation pump.
Attention: the most distant point of the pipeline is the corner room, therefore, the pressure here is the lowest
Concept of thermodynamic function. Internal energy, total energy of the system. The stability of the state of the system.
Other
parameters that depend on the main ones, called
TD
state functions systems.
In chemistry, the most commonly used are:
-
internal
energyUand
its change U
at V = const; -
enthalpy(heat content)
H
and its change H
for p = const; -
entropy
S
and its change S; -
energy
Gibbs G
and its change G
for p = const and T = const. -
For
state functions it is characteristic that their
change in chem. reaction is determined
only initial and final state
system and does not depend on the path or method
the course of the process.
Internal
energy (Internal Energy) - U.
Internal
energy U
is defined as the energy of random,
in disorderly motion
molecules. The energy of molecules is in
range from the high required for
movement, up to noticeable only with the help of
energy microscope on molecular or
atomic level.
-
Kinetic
energy of motion of the system as a whole -
Potential
positional energy
systems in an external field -
Internal
energy.
For
chem. reactions change in total energy
chem. systems are determined only by change
her inner energy.
Internal
energy includes translational,
rotational, vibrational energy
atoms of molecules, as well as the energy of motion
electrons in atoms, intranuclear
energy.
Quantity
internal energy (U)
substances is determined by the amount
substance, its composition and state
Sustainability
system is determined by the number
internal energy: the greater the internal
energy, the less stable the system
Stock
the internal energy of the system depends on
system state parameters, nature
in-va and is directly proportional to the mass
substances.
Absolute
determine the value of internal energy
impossible, because can't bring the system
into a state of complete emptiness.
Can
judge only the change in the internal
system energy U
during its transition from the initial state
U1
to final U2:
U
= U2U1,
The change
internal energy of the system (U),
as well as changing any TD function, defined
the difference between its values in the final and
initial states.
If
U2
U1,
then U
= U2U1
0,
if
U2
U1,
then U
= U2U1
0,
if
internal energy does not change
(U2
= U1),
then U
= 0.
In
in all cases, all changes are subject to
law
energy conservation:
Energy
does not disappear without a trace and does not arise
from nothing, but only passes from one
form to another in equivalent quantities.
Consider
system in the form of a cylinder with a movable
piston filled with gas
At
p = const heat Qp
goes to increase the stock of internal
energy U2
(U2U1)
U>0
and for the system to perform work (A) on
gas expansion V2
V1
and lift the piston.
Next,
QR=
U
+ A.