Introduction
In terms of geological reserves, the main energy raw material in Ukraine is coal, the reserves of which are about 120 billion tons, including explored ones - about 50 billion tons. according to various estimates up to 300-400 years. In Ukraine, the share of coal reserves in the fuel and energy balance is 94.5%, respectively, oil - 2% and gas - 3.6%. []
Fig 1. - Chemical structure of brown coal
The development of the Ukrainian economy is associated with the intensification of energy consumption, the main of which, in the absence of its own developed gas and oil industry, coal becomes uncontested. It is possible to increase its production only through a radical reconstruction and construction of new coal mines, mines, in turn, this requires a long time and large capital investments.
One of the ways to solve this problem is to expand the use of brown coal in large and small thermal power plants, which will contribute to a certain extent to stabilizing the country's fuel and energy balance and creating a reserve of time for the development of the coal industry.
How does the process of coal pyrolysis proceed?
As we mentioned earlier, the process of pyrolysis of coal is based on heating coals to a certain temperature without access to oxygen in order to thermally destroy it. During this process, the following groups of chemical reactions take place:
- Depolymerization of the organic mass of coal with the formation of organic molecules with a lower molecular weight
- Secondary reactions of transformations of products formed in the process of pyrolysis, including:
- condensation
- polymerization
- aromatization
- alkylation
Both groups of chemical reactions proceed both sequentially and in parallel. The final result of the totality of these thermochemical transformations is the formation of liquid gaseous and solid products.
It should be mentioned that coal pyrolysis is carried out in different temperature ranges. The choice of pyrolysis temperature depends on the type of products to be obtained in the end. Low-temperature pyrolysis (or semi-coking) is usually performed at 500 - 600 degrees Celsius, and high-temperature pyrolysis (or, as it is also called, coking) is performed at 900 - 1100 degrees Celsius.
Main products of coal
The most conservative estimates suggest that there are 600 items of coal products. Scientists have developed various methods for obtaining coal processing products. The processing method depends on the desired end product. For example, in order to obtain pure products, such primary products of coal processing - coke oven gas, ammonia, toluene, benzene - use liquid flushing oils. In special devices, products are sealed and protected from premature destruction. The processes of primary processing also involve the method of coking, in which coal is heated to a temperature of + 1000 ° C with completely blocked access to oxygen. At the end of all the necessary procedures, any primary product is additionally cleaned. The main products of coal processing:
- naphthalene
- phenol
- hydrocarbon
- salicylic alcohol
- lead
- vanadium
- germanium
- zinc.
Without all these products, our life would be much more difficult. Take the cosmetic industry, for example, it is the most useful area for people to use coal processing products. Such a coal processing product as zinc is widely used to treat oily skin and acne. Zinc, as well as sulfur, is added to creams, serums, masks, lotions and tonics.Sulfur eliminates existing inflammation, and zinc prevents the development of new inflammations. In addition, therapeutic ointments based on lead and zinc are used to treat burns and injuries. An ideal assistant for psoriasis is the same zinc, as well as clay products of coal. Coal is a raw material for the creation of excellent sorbents that are used in medicine to treat diseases of the intestines and stomach. Sorbents, which contain zinc, are used to treat dandruff and oily seborrhea. As a result of a process such as hydrogenation, liquid fuel is obtained from coal at enterprises. And the combustion products that remain after this process are an ideal raw material for a variety of building materials with refractory properties. For example, this is how ceramics are created.
|
Direction of use |
Brands, groups and subgroups |
|---|---|
|
1. Technological |
|
|
1.1. Layer coking |
All groups and subgroups of brands: DG, G, GZhO, GZh, Zh, KZh, K, KO, KSN, KS, OS, TS, SS |
|
1.2. Special pre-coking processes |
All coals used for layered coking, as well as grades T and D (subgroup DV) |
|
1.3. Producer gas production in stationary type gas generators: |
|
|
mixed gas |
Brands KS, SS, groups: ZB, 1GZhO, subgroups - DGF, TSV, 1TV |
|
water gas |
Group 2T, as well as anthracite |
|
1.4. Production of synthetic liquid fuels |
GZh brand, groups: 1B, 2G, subgroups - 2BV, ZBV, DV, DGV, 1GV |
|
1.5. semi-carbonization |
Brand DG, groups: 1B, 1G, subgroups - 2BV, ZBV, DV |
|
1.6. Production of carbonaceous filler (thermoanthracite) for electrode products and foundry coke |
Groups 2L, ZA, subgroups - 2TF and 1AF |
|
1.7. Production of calcium carbide, electrocorundum |
All anthracites, as well as a subgroup of 2TF |
|
2. Energy |
|
|
2.1. Pulverized and stratified combustion in stationary boiler plants |
Weight brown coals and athracites, as well as hard coals not used for coking. Anthracites are not used for flare-layer combustion |
|
2.2. Burning in reverberatory furnaces |
Brand DG, group i - 1G, 1SS, 2SS |
|
2.3. Combustion in mobile heat installations and use for communal and domestic needs |
Grades D, DG, G, SS, T, A, brown coals, anthracites and hard coals not used for coking |
|
3. Production of building materials |
|
|
3.1. Lime |
Marks D, DG, SS, A, groups 2B and ZB; grades GZh, K and groups 2G, 2Zh not used for coking |
|
3.2. Cement |
Grades B, DG, SS, TS, T, L, subgroup DV and grades KS, KSN, groups 27, 1GZhO not used for coking |
|
3.3. Brick |
Coals not used for coking |
|
4. Other productions |
|
|
4.1. Carbon adsorbents |
Subgroups: DV, 1GV, 1GZhOV, 2GZhOV |
|
4.2. active carbons |
ZSS group, 2TF subgroup |
|
4.3. Ore agglomeration |
Subgroups: 2TF, 1AB, 1AF, 2AB, ZAV |
Coal mining
People have long understood how important and irreplaceable it is, and the use of it was able to evaluate and adapt on such a scale relatively recently. Large-scale development of coal deposits began only in the XVI-XVII centuries. in England, and the extracted material was used mainly for the smelting of pig iron, necessary for the manufacture of cannons. But its production by today's standards was so insignificant that it cannot be called industrial.
Large-scale mining began only towards the middle of the 19th century, when the developing industrialization became indispensable for hard coal. Its use, however, at that time was limited exclusively to incineration. Hundreds of thousands of mines are now operating all over the world, producing more per day than in a few years in the 19th century.
Gravity enrichment
The gravitational method of coal enrichment is based on its different density and speed of movement in air or water.
The so-called wet enrichment process can be carried out on concentration tables, in heavy media, washing troughs, hydrocyclones, or by means of jigging on special machines.
The washing chute is a flat trough with low sides, which is placed at a slight slope.The pulp passes through the apparatus, the settled particles of coal are released through the discharge chamber of the chute. Now such devices are used very rarely due to low productivity.
Concentration tables are more suitable for beneficiation of high-sulphur coking coals and pyrite - types of coal not typical for Russia, therefore, they are practically not used in our country.
But jigging machines have become widespread. They separate the coal mixture into particles with different densities with the help of ascending and descending water flows moving in them at different speeds. Jigging is used for both small coals (12-0.5 mm) and large ones (10-12 mm).
This enrichment method is more efficient than other wet methods, except for enrichment in heavy liquids.
Heavy liquids are aqueous solutions of inorganic salts and mineral suspensions. Their density is higher than the density of coal, but at the same time less than the density of the primary rock. Therefore, coal, once in a solution or suspension, floats to the surface, and excess materials sink.
Concentrates obtained as a result of wet enrichment contain a lot of water, therefore, they are necessarily subjected to dehydration.
Dry beneficiation separates coal in air using other equipment such as dry trays, pneumatic separators or machines.
The material is fed onto the working surface of the equipment and
sorted under the action of an upward or pulsating air flow with
parallel shake. Coal grains depending on density and fineness
separated by moving in different directions.
Thanks to the enrichment, coal from the primary rock mass turns into a primary concentrate, the remaining rocks become waste.
Hydrotransportation of coal state of the problem
Hydraulic transportation of solid bulk materials was developed in the second half of the twentieth century. At present, pipeline transportation of oil, natural gas and oil products has become widespread. With the help of main hydrotransport systems, minerals and building materials, industrial waste and chemical raw materials are moved.
There are two fundamentally different technologies for the hydraulic transportation of coal.
The first technology is transportation in slurry with a mass concentration of C = 50%, followed by dehydration at the receiving terminal. Coal is crushed to a particle size of 0-1 (3-6) mm and mixed with water (the ratio of liquid and solid is 1: 1).
One of the first in the world is the main coal pipeline of the Black Mesa mine (Arizona, USA), 439 km long and with a capacity of 5.8 million tons / year. In 1964, the energy company Peabody Energy signed a contract with the Navajo and TAPI tribes to use their water resources to create slurry and transport it to the 790 MW Mohavi thermal power plant.
The process required large amounts of water, which caused an ecological crisis in these areas. Under the pressure of social and ethno-religious movements, the coal pipeline, despite its technological suitability and economic efficiency, was mothballed on December 31, 2005. p>
At the dewatering plant of the Black Mesa coal pipeline, the entire pulp mass was heated to 70 ° C, then dehydrated in centrifuges with a rotor diameter of 1000 mm and a rotation speed of 1000 min. The cake with a moisture content of 20% was subjected to thermal drying in mill-dryers. Heating the pulp before centrifugation reduced the moisture content of the cake from 28 to 20%. Centrifuge, which was 6.5% of the coal, or burned in the form of VVVS, or stored in a sludge tank. Due to the difficulty of obtaining HVVS in the first years of operation of the coal pipeline, a large amount of the solid phase of centrate was collected in the sludge pit, which posed a danger to the environment. P>
The second technology of hydraulic transportation of coal is in the form of highly concentrated water-coal suspensions (HVVS). [] At the receiving terminal, VVVS is used as a water-coal fuel (VUT). P>
The classic method of preparing BBVS consists of three main stages (Fig. 1.4):
- Crushing of run-of-mine coal to a fineness of 10 .. 20 mm;
- Wet grinding of coal (in the presence of water and plasticizer) up to 0.1-0.2 mm;
-
Homogenization, storage, transportation.
Rice. 1.4 - Scheme of preparation of VUT
For grinding, ball or rod drum mills with a special set of grinding bodies are used, which provides the desired binary granulometric composition of the coal phase. This stage is the key one in the preparation of CWF, since it determines the further characteristics of CWF (granulometric composition, viscosity, stability, etc.). In addition, this stage is usually the most energy-intensive.
At the stage of wet grinding, various additives necessary to increase the static stability of the WCF, reduce viscosity, and others can be included in the composition of the CWF.
Other recycling methods
To understand why oil is better than coal, you need to figure out what other treatments they are subjected to. Oil is processed through cracking, that is, the thermocatalytic transformation of its parts. Cracking can be one of the following types:
- Thermal. In this case, the splitting of hydrocarbons under the influence of elevated temperatures is carried out.
- Catalytic. It is carried out at high temperature, but a catalyst is also added, thanks to which you can control the process, as well as lead it in a certain direction.
If we talk about how oil is better than coal, then it should be said that in the process of cracking, organic substances that are widely used in the industrial synthesis are formed.
Varieties of hard coal
Deposits of coal seams can reach a depth of several kilometers, going into the thickness of the earth, but not always and not everywhere, because it is heterogeneous both in content and in appearance.
There are 3 main types of this fossil: anthracite, brown coal, and peat, which very remotely resembles coal.
Anthracite is the oldest formation of its kind on the planet, the average age of this species is 280,000,000 years. It is very hard, has a high density, and its carbon content is 96-98%.
The hardness and density are relatively low, as is the carbon content in it. It has an unstable, loose structure and is also oversaturated with water, the content of which in it can reach up to 20%.
Peat is also classified as a type of coal, but not yet formed, so it has nothing to do with coal.
Coal preparation
The miners ship the rock mined at the open pit or in the mine to special equipment, which delivers it to the mining and processing plant. There, the rock mass passes the initial stage of enrichment - preparation.
The primary rock is sorted into classes according to the size of the pieces and the presence of mineral inclusions. The main task is to identify carbon-containing components.
To separate the coal fractions of the GOFs, screening and crushing procedures are carried out on special equipment.
Screen for coal enrichment. Photo: 150tonn.ru
First, the rock is loaded into screens - devices in the form of one or more boxes with sieves or sieves with calibrated holes. Pieces of rock are sifted, and then sorted into fractions in classifiers.
All classifiers work approximately according to the same scheme: pulp (a mixture of coal and liquid) continuously flows into a vessel filled with water. Large particles of coal quickly settle to the bottom of the vessel, and small ones “leave” along with the pulp through the drain threshold.
Then the sorted rock is crushed to the required size using crushers.
The standard classification of coal size includes the following types: slab (more than 100 mm), large (50-100 mm), walnut (26-50 mm), small (13-25 mm), seed (6-13 mm), fine (less than 6 mm). There is also the so-called ordinary coal, which has unlimited dimensions.
Coal coking products
Coking coal is coal that, through industrial coking, makes it possible to obtain coke, which is of technical value. In the process of coking coal, their technical composition, coking capacity, sintering ability, and other characteristics are necessarily taken into account. How does the coal coking process proceed? Coking is a technological process that has specific stages:
- preparation for coking. At this stage, coal is crushed and mixed to form a charge (mixture for coking)
- coking. This process is carried out in the chambers of a coke oven using gas heating. The mixture is placed in a coke oven, where heating is carried out for 15 hours at a temperature of approximately 1000 °C.
- the formation of a "coke pie".
Coking is a set of processes that occur in coal when it is heated. At the same time, about 650-750 kg of coke is obtained from a ton of dry charge. It is used in metallurgy, used as a reagent and fuel in some branches of the chemical industry. In addition, calcium carbide is created from it. Qualitative characteristics of coke are flammability and reactivity. The main products of coal coking, in addition to coke itself:
- coke gas. About 310-340 m3 is obtained from a ton of dry coal. The qualitative and quantitative composition of coke oven gas determines the coking temperature. Direct coke oven gas comes out of the coke chamber, which contains gaseous products, coal tar vapors, raw benzene and water. If you remove the resin, raw benzene, water and ammonia from it, reverse coke oven gas is formed. It is it that is used as a raw material for chemical synthesis. Today, this gas is used as a fuel in metallurgical plants, in public utilities and as a chemical raw material.
- Coal tar is a viscous black-brown liquid that contains about 300 different substances. The most valuable components of this resin are aromatic and heterocyclic compounds: benzene, toluene, xylenes, phenol, naphthalene. The amount of resin reaches 3-4% of the mass of coking gas. About 60 different products are obtained from coal tar. These substances are raw materials for the production of dyes, chemical fibers, plastics.
- crude benzene is a mixture in which carbon disulfide, benzene, toluene, xylenes are present. The yield of crude benzene reaches only 1.1% of the mass of coal. In the distillation process, individual aromatic hydrocarbons and mixtures of hydrocarbons are isolated from crude benzene.
- concentrate of chemical (aromatic) substances (benzene and its homologues) is designed to create pure products that are used in the chemical industry, for the production of plastics, solvents, dyes
- tar water is a low concentrated aqueous solution of ammonia and ammonium salts, in which there is an admixture of phenol, pyridine bases and some other products. Ammonia is released from the tar water during processing, which, together with ammonia from coke gas, is used to produce ammonium sulfate and concentrated ammonia water.
|
Conventions |
Piece size limits |
||
|---|---|---|---|
|
Varietal |
|||
|
Large (fist) |
|||
|
Combined and eliminations |
|||
|
Large with slab |
|||
|
Nut with large |
|||
|
small walnut |
|||
|
seed with small |
|||
|
Seed with a lump |
|||
|
Small with seed and shtyb |
|||
|
Nut with small, seed and stump |
|||
List of sources
- Smirnov V. O., Sergeev P. V., Biletsky V. S. Technology of enrichment vugillya. Head helper. - Donetsk: Skhidny vydavnichiy dіm, - 2011. - 476 p.
- Chun - Zhu Li. Advances in the Science of Victorian Brown Coal - Book, 2004. - 459p.
- Saranchuk V.I., Ilyashov M.O., Oshovsky V.V., Biletsky V.S. Fundamentals of chemistry and physics of combustible copalins. (Pidruchnik with the signature stamp of the Ministry of Higher Education). - Donetsk: Skhіdniy vydavnichiy dіm, 2008. - 640 p.
- Svitly Yu.G., Biletsky V.S. Hydraulic transport (monograph).- Donetsk: Skhіdniy vydavnichiy dіm, Donetsk branch of NTSH, "Editorial staff of the encyclopedia", 2009. - 436 p.
- Small hand encyclopedia. v.1,2 / Ed. V. S. Biletsky. - Donetsk: "Donbas", 2004, 2007.
- Lipovich V.G., Kalabin G.A., Kalechits I.V. Chemistry and coal processing - Moscow: Chemistry, 1988. - 336 p.
- Chistyakov A.N. Handbook on chemistry and technology of solid fossil fuels. - St. Petersburg: publishing house. Synthesis Company. - 1996. - 363 p.
- Svyatec I.E., Agroskin A.A. Brown coals as a technological raw material. - M., Nedra, 1976. - 223 p.
- Khodakov G.S., Gorlov E.G., Golovin G.S. Production and pipeline transportation of suspension water-coal fuel// Chemistry of solid fuel. - 2006. - No. 4. - S. 22-39
- Krut O.A. - Kiev: Nauk. Dumka, 2002. - 172 p.
- Trainis V.V. Main pipelines in the USA // Coal. - 1978 - No. 11, p. 74-77.
- Biletsky V.S., Sergeev P.V., Papushin Yu.L. Theory and practice of selective oil aggregation of Vugill. Donetsk: MCP Gran, 1996. - 264 p.
- Gordeev G.P., Fedotova V.M. On the critical moisture content of brown coals// Chemistry of solid fuels. - 1989. - No. 6. – 76-78 p.
- Elishevich A.T., Ogloblin N.D., Beletsky V.S., Papushin Yu.L. Enrichment of ultrafine coals. - Donetsk, Donbas, 1986. - 64 p.
- Tamko V.O., Biletsky V.S., Shendrik T., Krasіlov O.O. Injection of mechanical detailing of the brown vug of the Oleksandrіysky family on yoga pіrolіz / / Donetsk Bulletin of the Scientific Association IM. Shevchenko. T. 21 - Donetsk: Skhіdny vydavnichiy dіm. - 2008. - S. 97-103.
- Kalechitsa I.V. Chemical substances from coal. - M.: Chemistry, 1980. - 616 p.
- Tverdov A.A., Zhura A.V., Nikishichev S.B. Perspective directions of coal use// Globus. - 2009. - No. 2. - S. 16-19.
- Lebedev NN Chemistry and technology of basic organic and petrochemical synthesis. - M.: Chemistry, 1988. - 592 p.
-
Krylova A.Yu., Kozyukov E.A. The state of the processes for obtaining synthetic liquid fuels based on the Fischer-Tropsch synthesis // Chemistry of Solid Fuels. - 2007. - No. 6. - S. 16-25.
- Energy & Environmental Research Center (EERC). . – Access mode: http://www.undeerc.org/default.aspx
- Boruk S.D., Winkler I.A., Makarova K.V. Having poured into the surface of the particles of the dispersed phase on the physical and chemical characteristics of water-boiled suspensions based on brown wool. - Science. Bulletin of ChNU. Vip. 453.: Chemistry. – Chernivtsi, 2009, p. 40-45.
- Kasatochkin V.I., Larina N.K. Structure and properties of natural coals. – M.: Nedra, 1975. – 158 p.
- Kegel K. Brown coal briquetting. - M., Ugletekhizdat, 1957. - 659 p.
-
Saranchuk V.I. Supramolecular organization, structure and properties of coal. - Kiev: Nauk. Dumka, 1988. - 190 p.
The use of coal in the modern world
Various uses of minerals. Coal was originally only a source of heat, then energy (it turned water into steam), but now, in this regard, the possibilities of coal are simply unlimited.
Thermal energy from coal combustion is converted into electrical energy, coke-chemical products are made from it, and liquid fuel is extracted. Hard coal is the only rock that contains such rare metals as germanium and gallium as impurities. From it, it is extracted, which is then processed into benzene, from which coumarone resin is isolated, which is used to manufacture all kinds of paints, varnishes, linoleum and rubber. Phenols and pyridine bases are obtained from coal. During processing, coal is used in the production of vanadium, graphite, sulfur, molybdenum, zinc, lead, and many more valuable and now irreplaceable products.
Coal is important for the national economy
Coal is one of the first minerals that man began to use as a fuel. Only at the end of the 19th century, other types of fuel began to gradually replace it: first oil, then products from it, later gas (natural and obtained from coal and other substances). Coal is widely used in the national economy. First of all, as fuel and chemical raw materials. For example, the metallurgical industry in the smelting of pig iron cannot do without coke. It is produced at coke-chemical enterprises from coal.
Where else is coal used?
Powerful thermal power plants in Russia and Ukraine (and not only) operate on the waste of coal mining (anthracite sludge).For the first time, metal was obtained using coke from iron ore in the 18th century in England. This in metallurgy was the beginning of the use of coal, more precisely, coke - a product of its processing. Prior to that, iron was obtained using charcoal, so in England in the 18th and 19th centuries almost the entire forest was cut down. The coking industry uses coal, processing it into coal coke and coke oven gas, and dozens of types of chemical products are produced (ethylene, toluene, xylenes, benzene, coke gasoline, resins, oils, and much more). Based on these chemical products, a wide variety of plastics, nitrogen and ammonia-phosphorus fertilizers, aqueous ammonia solutions (fertilizers), and plant protection chemicals are produced. They also produce detergents and washing powders, medicines for people and animals, solvents (solvents), sulfur or sulfuric acid, coumarone resins (for paints, varnishes, linoleum and rubber products), etc. A complete list of products of coke-chemical processing of coal takes up several pages.
How is the cost of coal?
Coconut charcoal - what is it?
One type of charcoal is coconut charcoal, which is made from the shells of nuts. It can be used in barbecues, grills, barbecues. It burns much longer than other charcoal, has no smell, no sulfur, and does not ignite from dripping grease. Purified coconut charcoal can be used for hookah, because when used it has neither smell nor taste. After special treatment (activation), the working surface of each piece of coal increases several times (and it becomes an excellent adsorbent). The use of coconut charcoal in water purification filters gives excellent results.
Final product
The resulting primary concentrate is subjected to refinement - in order to obtain a material that will fully comply with accepted standards. The final product with GOF is sent to consumers.
As a result, enrichment plants receive a concentrate that contains the largest amount of combustible mass with a minimum number of excess impurities. Due to this, the most important quality of the concentrate, the heat of combustion, increases.
Even in the process of enrichment, the so-called middling product is formed - a mixture of intergrowths of coal and rock components. In most cases, it is sent for re-enrichment, but sometimes it is sold as boiler fuel.
And the third product of coal preparation, which contains mainly rock minerals, is enrichment waste (otherwise they are called mixed). Some wastes contain enough coal for processing, so they are also sometimes sent for re-enrichment.
As a rule, coal enterprises store the remaining mixed mixes in tailings. But gradually, in the coal industry, the processing of coal-containing waste (for example, obtaining briquettes) is gaining ground.
Tags:coal enrichment
coal
3 Pyrolysis and gasification
Pyrolysis
Pyrolysis is the decomposition of brown coal when heated without air access. There are four main pyrolysis processes:
- semi-coking up to 500–550 °C;
- medium temperature coking 700–750 °С;
- high-temperature coking up to 900–1100°С;
-
graphitization 1300–3000 °С.
Brown coal does not soften when heated, and volatile substances are released, which partially decompose. In the residue, a more or less monolithic semi-coke is formed, which has undergone strong shrinkage. When semi-coking brown coal, three temperature zones are distinguished []: p>
- preheating zone up to 100°С;
- drying zone 100-125°C;
-
semi-coking zone 225-500°С.
During pyrolysis, under the influence of temperature, significant changes occur in coal. The first stage is the evaporation of moisture at temperatures up to 125-160 ° C, then the decomposition of the organic mass of brown coal begins.As the process proceeds, oxygen, hydrogen and nitrogen are removed, and the solid residue is enriched with carbon. At the initial stages, at temperatures up to 200 °C, oxygen is released mainly in the form of carbon dioxide and pyrogenetic water due to the elimination of functional groups, accompanied by condensation reactions of radicals that remain.
Nitrogen is released in the form of ammonia, other nitrogenous compounds and in the free state.
At a temperature of 200-350 ° C, a gradual decrease in the solid residue occurs, the release of vapors and gases increases only by 6-7%. The zone from 350 to 450 °C is characterized by an increase in the rate of release of the vapor-gas phase and a sharper decrease in the yield of solid residue. In the temperature range of 450-550 °C, there are small changes in the yield of both the solid residue and the vapor-gas mixture.
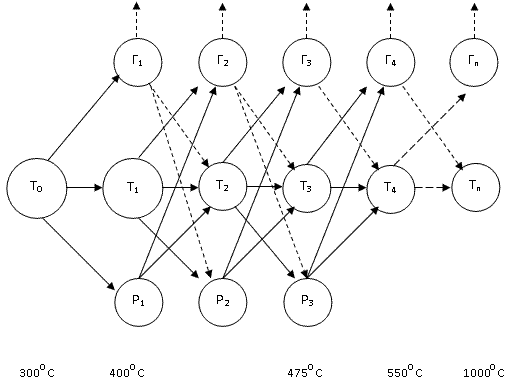
Schematic representation of the pyrolysis process Figure 1.3. []
Rice. 1.3 - Block diagram of the pyrolysis process
Gasification
The process of converting the organic mass of coal into gaseous substances is called gasification. In the process of gasification, carbon more often turns into carbon monoxide, hydrogen into water vapor and, together with sulfur, which is in the organic mass of coal, into hydrogen sulfide, nitrogen into nitrogen oxides. The mineral part of coal, depending on the gasification temperature, passes into ash or slag.
Coal gasification underlies many technological processes associated with its use. The first gasification processes were developed to produce combustible gases from coal, which were used as household fuel for street lighting, as industrial fuel for various high-temperature processes.
Before these processes, brown coal is crushed and, if necessary, dehydrated.
It is very important to bring brown coal to the required size - it can be gasification of lump (> 3mm), fine (1-3mm) and fine (7]
Requirements for brown coal, which is fed for pyrolysis and gasification
The rational moisture content of the initial coal for the pyrolysis process is moisture (Wrt) up to 15%, ash content (Ad) up to 10%, coal should be low-sulfur. For the gasification process - moisture (Wrt) up to 65%, ash content (Ad) up to 40%. p>
conclusions
One of the directions of technical progress is the development of pipeline transport. The industrial and main hydrotransport of oil and bulk materials has the greatest prospects. Hydrotransport is characterized by continuity and uniformity of cargo flow, increased reliability, the possibility of full automation, independence from weather conditions, and has an economic advantage over rail transport, especially when the mines are located in remote areas; creates less noise, has significantly lower transport losses and man-made impact on the environment; short construction time.
There are several ways to hydraulically transport coal:
- slurry pipeline with further dehydration;
- transportation of highly concentrated water-coal fuel.
The negative properties of brown coal impede the use of hydrotransport; to solve this problem, a technology for treating coal with apolar reagents - oil aggregation was proposed. P>
Oil aggregation of coal is understood as a set of processes for structuring a thin polydisperse coal phase (grain size up to 3-5 mm) in an aqueous medium using oil reagents. These processes are based on the mechanism of adhesive interaction of the oleophilic coal surface with oils, which results in its selective wetting and aggregation in a turbulent water flow. Hydrophilic particles are not wetted by oil and are not included in aggregates, which allows them to be isolated in the form of a rock suspension. P>
Based on the foregoing, for the upgrading of brown coal during its hydrotransportation, we have chosen the technology of oil coal aggregation, which is well combined with the technologies for its further processing and use: briquetting, liquefaction, gasification, pyrolysis. P>