How to do the calculation
Under normal atmospheric conditions and a temperature of 15°C, the density of propane in the liquid state is 510 kg/m3, and that of butane is 580 kg/m3. Propane in a gaseous state at atmospheric pressure and a temperature of 15 ° C is 1.9 kg / m3, and butane - 2.55 kg / m3. Under normal atmospheric conditions and a temperature of 15°C, 0.392 m3 of gas is formed from 1 kg of liquid butane, and 0.526 m3 from 1 kg of propane.
Knowing the volume of a gas and its specific gravity, we can determine its mass. So, if the estimate indicates 27 m 3 of technical propane-butane, then multiplying 27 by 2.25 we find out that this volume weighs 60.27 kg. Now, knowing the density of liquefied gas, you can calculate its volume in liters or cubic decimeters. The density of propane-butane in a ratio of 80/20 at a temperature of 10 C is 0.528 kg/dm 3 . Knowing the formula for the density of a substance (mass divided by volume), we can find the volume of 60.27 kg of gas. It is 60.27 kg / 0.528 kg / dm 3 \u003d 114.15 dm 3 or 114 liters.
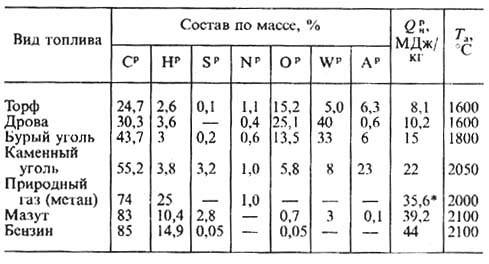
Composition and characteristics of fuels
Any substance capable of releasing a significant amount of heat during combustion (oxidation) can be called a fuel. According to the definition given by D. I. Mendeleev, “fuel is a combustible substance deliberately burned to produce heat.”
The tables below show the main characteristics of various types of fuels: composition, lower heating value, ash content, moisture content, etc.
Approximate composition and thermal characteristics of the combustible mass of solid fuel
| Fuel | The composition of the combustible mass,% | Yield of volatile substances, VG, % | Lower calorific value, MJ/kg | Heat output, tmax, °C | RO2 max* combustion products, % | ||||
| SG | SG | HG | OG | NG | |||||
| Firewood | 51 | — | 6,1 | 42,2 | 0,6 | 85 | 19 | 1980 | 20,5 |
| Peat | 58 | 0,3 | 6 | 33,6 | 2,5 | 70 | 8,12 | 2050 | 19,5 |
| oil shale | 60—75 | 4—13 | 7—10 | 12—17 | 0,3—1,2 | 80—90 | 7,66 | 2120 | 16,7 |
| Brown coal | 64—78 | 0,3—6 | 3,8—6,3 | 15,26 | 0,6—1,6 | 40—60 | 27 | — | 19,5 |
| Coal | 75—90 | 0,5—6 | 4—6 | 2—13 | 1-2,7 | 9—50 | 33 | 2130 | 18,72 |
| Semi-anthracite | 90—94 | 0,5—3 | 3—4 | 2—5 | 1 | 6—9 | 34 | 2130 | 19,32 |
| Anthracite | 93—94 | 2—3 | 2 | 1—2 | 1 | 3—4 | 33 | 2130 | 20,2 |
* - RO2 = CO2 + SO2
Characteristics of liquid fuels derived from petroleum
| Fuel | The composition of the combustible mass,% | Ash content of dry fuel, AC, % | Moisture of working fuel, WP, % | Lower calorific value of working fuel, MJ/kg | |||
| Carbon SG | Hydrogen NG | Sulfur SG | Oxygen and NitrogenO + NG | ||||
| Petrol | 85 | 14,9 | 0,05 | 0,05 | 43,8 | ||
| Kerosene | 86 | 13,7 | 0,2 | 0,1 | 43,0 | ||
| diesel | 86,3 | 13,3 | 0,3 | 0,1 | Footprints | Footprints | 42,4 |
| Solar | 86,5 | 12,8 | 0,3 | 0,4 | 0,02 | Footprints | 42,0 |
| Motor | 86,5 | 12,6 | 0,4 | 0,5 | 0,05 | 1,5 | 41,5 |
| Low-sulfur fuel oil | 86,5 | 12,5 | 0,5 | 0,5 | 0,1 | 1,0 | 41,3 |
| Sulphurous fuel oil | 85 | 11,8 | 2,5 | 0,7 | 0,15 | 1,0 | 40,2 |
| Heavy fuel oil | 84 | 11,5 | 3,5 | 0,5 | 0,1 | 1,0 | 40,0 |
The fuel in the form in which it enters for combustion in furnaces or internal combustion engines is called working fuel.
The name "combustible mass" is conditional, since only carbon, hydrogen and sulfur are its really combustible elements. The combustible mass can be characterized as a fuel that does not contain ash and is in a completely dry state.
Ash content of fuel. Ash is a solid non-combustible residue remaining after the combustion of fuel in an air atmosphere. Ash can be in the form of loose mass with an average density of 600 kg/m3 and in the form of fused plates and lumps, called slags, with a density of up to 800 kg/m3.
The moisture content of the fuel is determined according to GOST 11014-2001 by drying the sample at 105 - 110 °C. The maximum humidity reaches 50% or more and determines the economic feasibility of using this fuel. Moisture reduces the temperature in the furnace and increases the volume of flue gases.
Composition and heat of combustion of combustible gases
| Name of gas | Composition of dry gas, % by volume | Net calorific value of dry gas Qns, MJ/m3 | |||||||
| CH4 | H2 | CO | CnHm | O2 | CO2 | H2C | N2 | ||
| Natural | 94,9 | — | — | 3,8 | — | 0,4 | — | 0,9 | 36,7 |
| Coke (refined) | 22,5 | 57,5 | 6,8 | 1,9 | 0,8 | 2,3 | 0,4 | 7,8 | 16,6 |
| Domain | 0,3 | 2,7 | 28 | — | — | 10,2 | 0,3 | 58,5 | 4,0 |
| Liquefied (estimated) | 4 | Propane 79, ethane 6, isobutane 11 | 88,5 |
The lower calorific value of a working fuel is the heat released during the complete combustion of 1 kg of fuel, minus the heat spent on the evaporation of both the moisture contained in the fuel and the moisture generated from the combustion of hydrogen.
The higher calorific value of a working fuel is the heat released during the complete combustion of 1 kg of fuel, assuming that the water vapor formed during combustion condenses.
How many cubes of saturated steam are in one gigacalorie. How to convert gigacalories to cubic meters
is the temperature of the heat carrier in the return pipeline.
Determine the speed of the water in the pipe
The speed of water movement is determined by the formula: V (m/s) = 4Q/π D2,
where: Q - water flow in m3 / s; π = 3.14;
D is the diameter of the pipeline in m2;
Calculation example: Water consumption Q = 5 m3 / h = 5 m3 / 3600 s = 0.001388 m3 / s; Pipe DN = 50 mm = 0.05 m;
V \u003d 4 * 0.001388 / 3.14 * 0.005 * 0.005 \u003d 0.707 m / s
When calculating systems, Du (nominal diameter) of the pipeline is determined from the condition,
that the average speed of the coolant in the locking devices, in order to avoid water hammer when closing, should not exceed 2 m / s.
The speed of the coolant in the pipes of water heating systems should be taken depending on the permissible sound level:
— no more than 1.5 m/s in public buildings and premises;
- no more than 2 m / s in administrative buildings and premises;
— no more than 3 m/s in industrial buildings and premises.
(minimum speed of water movement from the condition of air removal V = 0.2-0.3 m/s)
Heating equipment for heating with liquefied gas
The liquefied gas boiler is characterized by safe design and reliable operation.
For heating a private house on liquefied gas, both heating boilers with a water circuit and gas convectors are used. But among all types of such equipment, liquefied gas heating boilers are still in the lead, as the most productive. Reviews of heating on liquefied gas using convectors are rarely positive.
Gas heating boilers for liquefied gas in their design are almost the same as those that consume main gas. The only difference is in the design of the burners, since the pressure of propane-butane coming from the cylinder is almost 2 times higher than that of natural methane. Accordingly, the jets in the burners also differ in inner diameter. There are also some differences in the devices for adjusting the air supply.
Gas heating boilers for liquefied gas in their design are almost the same as those that consume main gas. The only difference is in the design of the burners, since the pressure of propane-butane coming from the cylinder is almost 2 times higher than that of natural methane. Accordingly, the jets in the burners also differ in inner diameter. There are also some differences in the devices for adjusting the air supply.
The structural differences are so small that, if necessary, it is enough just to replace the burners in a boiler designed for methane, and you do not have to buy a new heating boiler for liquefied gas.
Consider how the main models of boilers for a liquefied gas heating system differ from each other:
- Boiler type. Among the units for heating a private house with liquefied gas in cylinders, single-circuit and double-circuit boilers are distinguished. The former serve only for the heating system, while the latter, in addition, provide hot water. The combustion chamber in boilers is arranged differently; it can be open or closed. Both large floor models and compact wall models are produced;
- efficiency. Judging by the reviews, liquefied gas heating can become truly rational and economical if the gas boiler has an efficiency of at least 90-94%;
- Boiler power. It is considered one of the main parameters for heating a private house with liquefied gas. It is necessary to make sure that the passport characteristics of the unit will allow it to develop sufficient power to provide the entire area of \u200b\u200bthe dwelling with heat, but at the same time avoiding excessive consumption of liquefied gas for heating;
- Manufacturer. While piping in a liquefied gas heating system can be done by hand, a gas boiler should by no means be homemade.Moreover, it is desirable to give preference to well-established domestic or foreign manufacturers.
Liquefied gas boilers are forbidden to be installed in basements, since the propane-butane mixture is heavier than air. Such gas does not escape during leaks, but accumulates at floor level, which can lead to an explosion.
Fuel combustion heat
Any fuel, when burned, releases heat (energy), quantified in joules or calories (4.3J = 1cal). In practice, to measure the amount of heat that is released during the combustion of fuel, calorimeters are used - complex devices for laboratory use. The heat of combustion is also called the calorific value.
The amount of heat obtained from the combustion of fuel depends not only on its calorific value, but also on its mass.
To compare substances in terms of the amount of energy released during combustion, the value of the specific heat of combustion is more convenient. It shows the amount of heat generated during the combustion of one kilogram (mass specific heat of combustion) or one liter, cubic meter (volume specific heat of combustion) of fuel.
The units of specific heat of combustion of fuel accepted in the SI system are kcal / kg, MJ / kg, kcal / m³, MJ / m³, as well as their derivatives.
The energy value of fuel is determined precisely by the value of its specific heat of combustion. The relationship between the amount of heat generated during the combustion of fuel, its mass and the specific heat of combustion is expressed by a simple formula:
Q = q m, where Q is the amount of heat in J, q is the specific heat of combustion in J/kg, m is the mass of the substance in kg.
For all types of fuel and most combustible substances, the values of the specific heat of combustion have long been determined and tabulated, which are used by specialists when calculating the heat released during the combustion of fuel or other materials. In different tables, slight discrepancies are possible, obviously explained by slightly different measurement methods or different calorific value of the same type of combustible materials extracted from different deposits.
Specific heat of combustion of some types of fuel
Of the solid fuels, coal has the highest energy intensity - 27 MJ / kg (anthracite - 28 MJ / kg). Charcoal has similar indicators (27 MJ / kg). Brown coal is much less calorific - 13 MJ / kg. In addition, it usually contains a lot of moisture (up to 60%), which, evaporating, reduces the value of the total calorific value.
Peat burns with a heat of 14-17 MJ / kg (depending on its condition - crumb, pressed, briquette). Firewood dried to 20% moisture emits from 8 to 15 MJ/kg. At the same time, the amount of energy received from aspen and from birch can almost double. Approximately the same indicators are given by pellets from different materials - from 14 to 18 MJ / kg.
Much less than solid fuels, liquid fuels differ in specific heat of combustion. Thus, the specific heat of combustion of diesel fuel is 43 MJ/l, gasoline is 44 MJ/l, kerosene is 43.5 MJ/l, fuel oil is 40.6 MJ/l.
The specific heat of combustion of natural gas is 33.5 MJ/m³, propane - 45 MJ/m³. The most energy-intensive gaseous fuel is hydrogen gas (120 MJ/m³). It is very promising for use as a fuel, but to date, optimal options for its storage and transportation have not yet been found.
Comparison of the energy intensity of various types of fuel
When comparing the energy value of the main types of solid, liquid and gaseous fuels, it can be established that one liter of gasoline or diesel fuel corresponds to 1.3 m³ of natural gas, one kilogram of coal - 0.8 m³ of gas, one kg of firewood - 0.4 m³ of gas.
The calorific value of fuel is the most important indicator of efficiency, but the breadth of its distribution in the areas of human activity depends on the technical capabilities and economic indicators of use.
Natural gas and its calorific value
Feature of fossil fuel
Ecologists believe that gas is the cleanest fuel; when burned, it releases much less toxic substances than wood, coal, and oil. This fuel is used daily by people and contains such an additive as an odorant, it is added at equipped installations in a ratio of 16 milligrams per 1,000 cubic meters of gas.
An important component of the substance is methane (approximately 88-96%), the rest is other chemicals:
The amount of methane in natural fuel directly depends on its field.
Deposit types
Several types of gas deposits are noted. They are divided into the following types:
Their distinguishing feature is the hydrocarbon content. Gas deposits contain approximately 85–90% of the presented substance, oil fields contain no more than 50%. The remaining percentages are occupied by substances such as butane, propane and oil.
A huge disadvantage of oil generation is its flushing from various kinds of additives. Sulfur as an impurity is exploited at technical enterprises.
Natural gas consumption
Butane is consumed as fuel at gas stations for cars, and an organic substance called "propane" is used to fuel lighters. Acetylene is a highly flammable substance and is used in welding and cutting metal.
Fossil fuel is used in everyday life:
This kind of fuel is considered the most budgetary and harmless, the only drawback is the emission of carbon dioxide during combustion into the atmosphere. Scientists all over the planet are looking for a replacement for thermal energy.
Calorific value
The calorific value of natural gas is the amount of heat generated with sufficient burnout of a unit of fuel. The amount of heat released during combustion is referred to one cubic meter, taken in natural conditions.
The thermal capacity of natural gas is measured in the following terms:
There is a high and low calorific value:
- High. Considers the heat of water vapor that occurs during the combustion of fuel.
- Low. It does not take into account the heat contained in water vapor, since such vapors do not lend themselves to condensation, but leave with combustion products. Due to the accumulation of water vapor, it generates an amount of heat equal to 540 kcal / kg. In addition, when the condensate cools, heat from 80 to one hundred kcal / kg is released. In general, due to the accumulation of water vapor, more than 600 kcal / kg are formed, this is the distinguishing feature between high and low heat output.
If the calorific value of natural gas is less than 3500 kcal / Nm 3, it is more often used in industry. It does not need to be transported for long distances, and it becomes much easier to carry out combustion. Serious changes in the calorific value of the gas require frequent adjustment and sometimes replacement of a large number of standardized burners of household sensors, which leads to difficulties.
This situation leads to an increase in the diameter of the gas pipeline, as well as an increase in the cost of metal, laying networks and operation. The big disadvantage of low-calorie fossil fuels is the huge content of carbon monoxide, in connection with this, the level of danger increases during the operation of the fuel and during the maintenance of the pipeline, in turn, as well as equipment.
The heat released during combustion, not exceeding 3500 kcal/nm 3 , is most often used in industrial production, where it is not necessary to transfer it over a long distance and easily form combustion.
Accounting for gas consumption without the use of meters
Gas can be used in everyday life in three ways and, depending on the purpose, the following units of measurement are used:
- for cooking and heating water - for each person registered in the room (cubic meters / person);
- for heating a dwelling during the heating period (from October to April) - per 1 square meter of the total area (cub.m / sq.m).
The annex to Government Decree No. 373 of 13.06.2006 indicates the minimum allowable gas consumption standards for the population in residential premises in which metering devices are not installed.
Gas consumption standards for 1 person without a meter by region
Let us give the indicators of the standard by region using the example of consumption of 1 cubic meter per person from July 1, 2019. You can learn more about each by downloading the document file.
Today, the standard for natural gas without a meter, taking into account cooking and heating water using a gas stove in the presence of central heating and central hot water supply, is as follows:
| Region | Standard (1 cubic meter/person) | All regulations |
|---|---|---|
| Moscow and Moscow region | 10 | more |
| St. Petersburg and Leningrad region | 13 | more |
| Yekaterinburg and Sverdlovsk region | 10,2 | more |
| Krasnodar region | 11,3 | more |
| Novosibirsk region | 10 | more |
| Omsk and Omsk region | 13,06 | more |
| Perm region | 12 | more |
| Rostov-on-Don and Rostov region | 13 | more |
| Samara and Samara region | 13 | more |
| Saratov and Saratov region | 11,5 | more |
| Crimea | 11,3 | more |
| Nizhny Novgorod and Nizhny Novgorod region | 11 | more |
| Ufa and the Republic of Bashkortostan | 12 | more |
In private households, gas can be used to heat both residential and non-residential buildings. Baths, greenhouses, garages, etc. are non-residential. If there is a private economy, the consumption of the resource is taken into account depending on the number of livestock units and their type. Per head per month:
- horses - 5.2 - 5.3 m3;
- cows - 11.4 - 11.5 m3;
- pigs - 21.8 - 21.9 m3.
Therefore, in the absence of metering devices, a fee is charged based on the following parameters:
- the number of square meters of residential and non-residential area heated by gas;
- availability, type and number of livestock;
- the number of citizens registered in the premises (registered permanently and temporarily are taken into account);
- the degree of improvement, taking into account the connection to the central hot water supply networks.
For example, you can use the calculator and calculate the cost of gas costs with and without a meter.
Gas tariffs in 2019 with and without a meter
The amount of gas tariffs for the population increases annually. Although this is not as noticeable as for housing and communal services in general, but in comparison with previous years, the amounts have changed significantly. Since July 1, 2019, the price of natural gas with and without a meter in Russia has increased by 1.5% from the current ones.
Today, in the regions of Russia, the following gas prices apply for rooms where there are no metering devices in the presence of a gas stove and centralized hot water supply:
| Region | Tariff (rubles per 1 cubic meter) | All rates |
|---|---|---|
| Moscow and Moscow region | 6,83 | more |
| St. Petersburg (SPB) / Leningrad region | 6,37/6,60 | more |
| Yekaterinburg and Sverdlovsk region | 5,19 | more |
| Krasnodar / Krasnodar Territory | 5,48/6,43 | more |
| Novosibirsk region | 6,124 | more |
| Omsk and Omsk region | 8,44 | more |
| Perm region | 6,12 | more |
| Rostov-on-Don and Rostov region | 6,32 | more |
| Samara and Samara region | 7,48 | more |
| Saratov and Saratov region | 9,20 | more |
| Republic of Crimea |
|
more |
| Nizhny Novgorod and Nizhny Novgorod region | 6,11 | more |
| Ufa and the Republic of Bashkortostan | 7,20 | more |
Let's summarize:
- regulations differ depending on domestic gas use;
- the normative value is calculated for one citizen registered in the premises, or for 1 sq.m. heated living area;
- minimum tariffs are set for gas, applied in case of consumption of the resource within the monthly norm;
- in case of exceeding the normative consumption, increased tariffs are applied.
Watch an interesting video on how you can save on gas bills. What is better payment according to the standard or according to the meter?
How much m3 in a cylinder
Let's calculate the weight of the propane-butane mixture in the most common cylinder in construction: a volume of 50 with a maximum gas pressure of 1.6 MPa. The proportion of propane according to GOST 15860-84 must be at least 60% (note 1 to table 2):
50l \u003d 50dm3 \u003d 0.05m3;
0.05m3 • (510 • 0.6 + 580 •0.4) = 26.9kg
But due to the limitation of gas pressure of 1.6 MPa on the walls, more than 21 kg are not filled into a cylinder of this type.
Let's calculate the volume of the propane-butane mixture in the gaseous state:
21kg • (0.526 • 0.6 + 0.392 •0.4) = 9.93m3
Conclusion (for the case under consideration): 1 cylinder = 50l = 21kg = 9.93m3
Example: It is known that in a cylinder of 50 liters 21 kilograms of gas are filled, for which the test density is 0.567. To calculate liters, you need to divide 21 by 0.567. It turns out 37.04 liters of gas.
«>

adblock detector
Control valve calculation
Kv (Kvs) of the valve - characteristic of the valve capacity, there is a conditional volume flow of water through a fully open valve, m3 / h at a pressure drop of 1 bar under normal conditions. The specified value is the main characteristic of the valve.
, where G is the liquid flow rate, m3/h;
Δp - pressure drop across a fully open valve, bar
When selecting a valve, the Kv value is calculated, then rounded up to the nearest value corresponding to the passport characteristic (Kv) of the valve. Control valves are usually produced with Kvs values that increase exponentially:
Kvs: 1.0, 1.6, 2.5, 4.0, 6.3, 10, 16 …………
Calculate the radiator
Accurate thermal calculation is carried out using special methods.
An approximate calculation of the required thermal power for central Russia can be calculated using the following formula:
Power kW. = (Ld * Lsh * Hv) / 27,
where: Ld is the length of the room, m; Lsh - room width, m; Hv - ceiling height, m.
When narahuvanni schomisyachnyh payments for scorching that hot water is often blamed swindler. For example, as if in a bagatokvartirny booth there is a heating plant, then a heating plant with a supplier of thermal energy is carried out for saving gigacalories (Gcal). Vodnochay tariff for hot water for meshkantsiv sound set in rubles per cubic meter (m3). Schob rozіbratisya in payments, it is necessary to transfer Gcal to cubic meters.
Instruction
1
It is necessary to know that thermal energy, as it is reduced to Gcal, and water, which is measured in cubic meters, is absolutely different physical quantities. Tse vіdomo z the course of physics of the middle school. Therefore, it’s true that I’m not talking about the conversion of gigacalories to cubic meters, but about the significance of the availability of heat, we’ll glass it on hot water, and we’ll completely remove hot water.
2
By definition, a calorie is the amount of heat required to heat one cubic centimeter of water by 1 degree Celsius. A gigacalorie, zastosovuvana for the world of thermal energy in the heat and power industry and the communal state, is a billion calories. There are 100 centimeters in 1 meter, and in one cubic meter - 100 x 100 x 100 \u003d 1,000,000 centimeters. In this way, in order to heat the cube of water by 1 degree, it will take a million calories or 0.001 Gcal.
3
The temperature of the hot water flowing from the tap must be no less than 55°C. If the water at the entrance to the boiler room is cold and has a temperature of 5°C, then it will need to be heated by 50°C. 0.05 Gcal will be required for the production of 1 cubic meter. However, in Russia, running through pipes will inevitably blame the heat losses, and the amount of energy, consumption for the safety of the GWP, in operation it will be approximately 20% more. The average standard for the reduction of thermal energy for the production of a cube of hot water is taken equal to 0.059 Gcal.
4
Let's look at a simple example. Let it be in the middle period, if all the heat goes only to the security of the GVP, the consumption of thermal energy for the indications of the heat-filled lichnik is 20 Gcal per month, and the sacks, in the apartments of which water dispensers are installed, have consumed 30 cubic meters of hot water. They fall 30 x 0.059 = 1.77 Gcal.Heat output on all other bags (high їх will be 100): 20 - 1.77 \u003d 18.23 Gcal.
How to save
The financial costs of maintaining a comfortable microclimate in the house can be reduced by :
- additional insulation of all structures, installation of double-glazed windows and door structures without cold bridges;
- installation of high-quality supply and exhaust ventilation (incorrectly executed system can cause increased heat loss);
- use of alternative energy sources - solar panels, etc.
Separately, it is worth paying attention to the advantages of a collector heating system and automation, thanks to which an optimal temperature level is maintained in each of the rooms. This allows you to reduce the load on the boiler and fuel consumption when it gets warm outside, to reduce the heating of the coolant that is supplied to radiators or the underfloor heating system in unused rooms
If the house has a standard radiator system, a sheet of thin foamed heat insulator with an outer foil surface can be glued to the wall behind each heating device. Such a screen effectively reflects heat, preventing it from escaping through the wall into the street.
A set of measures aimed at improving the thermal efficiency of the house will help to minimize energy costs.
How to avoid heat loss
Fuel consumption for heating a house depends on the total area of the heated premises, as well as the heat loss coefficient. Any building loses heat through the roof, walls, window and door openings, the floor of the lower floor.
Respectively, the level of heat loss depends on the following factors :
- climate features;
- wind roses and the location of the house relative to the cardinal points;
- characteristics of the materials from which building structures and roofs are erected;
- the presence of a basement / basement;
- quality of floor insulation, wall structures, attic floors and roofs;
- number and tightness of door and window structures.
The thermal calculation of the house allows you to choose boiler equipment with optimal power parameters. In order to determine the need for heat as accurately as possible, the calculation is performed for each heated room separately. For example, the heat loss coefficient is higher for rooms with two windows, for corner rooms, etc.
Note! The power of the boiler is selected with some margin relative to the calculated values obtained. The boiler unit wears out faster and fails if it regularly works at the limit of its capabilities.
At the same time, an excessive power reserve turns into an increase in financial costs for the purchase of a boiler and increased fuel consumption.