Comparative characteristics
The basis of bronze and brass, as mentioned above, is the same metal - copper. The difference between these alloys lies in their chemical composition and, accordingly, in the characteristics that they possess. Naturally, the differences between these copper alloys also determine the scope of their application.
Due to the fact that bronze is a stronger and more durable material when compared with brass, bells, sculptural compositions, elements of fences, landscape and interior structures have been made from this material since ancient times. It is also important that many grades of this alloy are characterized by good fluidity in the molten state. This makes it possible to cast products even of very complex configuration from them. By adding various chemical elements to the chemical composition of bronze, it is possible to change its color in a fairly wide range, which is also of great importance in the production of decorative items.
This watch ring, judging by the color, is more likely yellow brass (bronze would be redder). Scratches easily remain on the surface - also a sign of brass
Brass differs from bronze in higher ductility and, accordingly, lower strength and wear resistance, which limits the use of this alloy in many areas. In addition, brass is less resistant to aggressive environments, in particular, salty sea water, which does not allow the use of brass products in shipbuilding, where bronze is used very actively and successfully.
There is also a noticeable difference in the color of these alloys and in their internal structure. Any experienced specialist can tell you how to distinguish brass from bronze: just look at the fracture of products made from these alloys. Brass at the break has a lighter color and a pronounced fine grain structure, while bronze is easily identified by the dark brown color of the break and the coarse internal structure.
Broken bronze sleeve
- The main alloying element in bronze is tin, while in brass it is zinc. At the same time, both alloys are created on the basis of one metal - copper.
- Bronze (even with a classic chemical composition) perfectly resists the effects of aggressive environments, in particular salty sea water. In order to improve the corrosion resistance of brass, it is necessary to introduce additional alloying elements into such an alloy.
- The strength and anti-friction characteristics of bronze are also better than those of brass. Such qualities significantly expand the scope of which not only strong and durable decorative elements are made, but also critical parts for use in various industries. Brass is more often used for the production of bimetallic elements ("steel - brass"), which demonstrate high resistance to the formation and development of corrosion processes.
- Bronze products have a dark brown color and coarse grain at the break, while brass products have a yellow-golden and fine-grained structure. Such a difference in color and internal structure makes it easy to determine which alloy the product is made of.
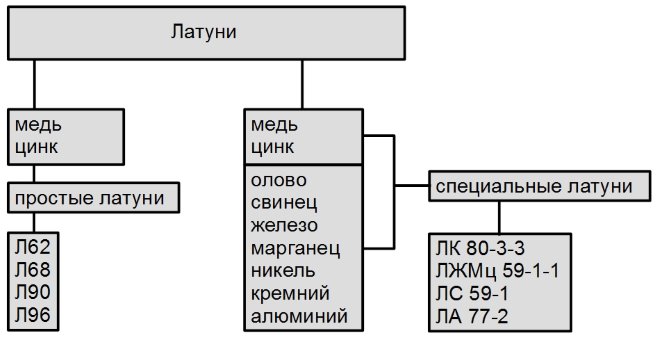
- Bronze, like brass, although they are based on a metal such as copper, are divided into completely different categories. So, bronze can be tin or tinless, while brass can be two- or multi-component.
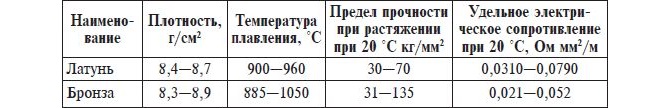
Comparison of properties of brass and bronze
Bronze and brass, whose melting point is lower than copper, can be used to make various items at home. However, for this, of course, it is necessary to stock up on appropriate equipment and study the technology and rules for performing such a technological operation as casting well.
Chemical composition and features of the internal structure
In order to understand well the characteristics of brass, it is important to understand what properties the chemical elements of which it is composed have. Such elements, as mentioned above, are copper and zinc.
Classification of brasses by chemical composition
Copper is one of the first metals that people began to use for the manufacture of products for various purposes. This element, which is included in the 11th group of the IV period of the periodic table, has atomic number 29 and is designated as Cu (short for Cuprum). Copper, which is a transition metal, is highly ductile and has a beautiful light golden color. When an oxide film is formed, the metal acquires an equally beautiful yellowish-red hue.
Zinc - the second main element in the chemical composition of brass - is also a metal that, unlike copper, does not occur in nature in its pure form. Zinc, which has atomic number 30, is included in the side subgroup of the 2nd group of the IV period of the periodic table. This metal, which began to be produced in the 12th century in India, is highly brittle under normal conditions. Without the oxide film that appears on the metal when it interacts with open air, its surface has a light blue color. This metal is designated by the symbol Zn (short for Zincum).
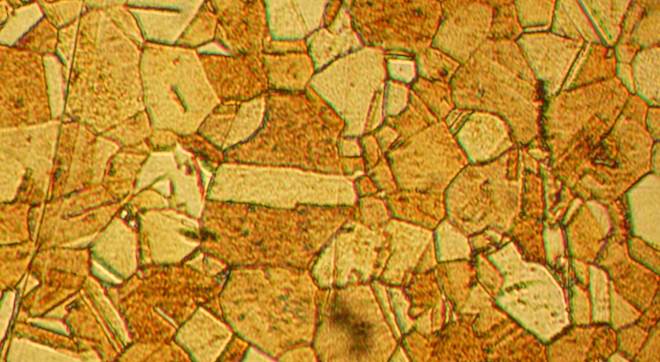
This is what the microstructure of a polished brass surface looks like under 400x magnification
The structure of brass, depending on the content of the main components in its composition, can consist of one α- or simultaneously α + β-phases. Such states, which the internal structure of the alloy can take, are distinguished by the following features:
- α-phase is a solution of copper and zinc, characterized by high stability, in which the molecules of the base metal (copper) have a face-centered cubic lattice;
- α + β-phase is also a stable solution, in which copper and zinc are contained in a ratio of 3: 2 (in such a solution, copper molecules have a simple unit cell).
The microstructure of α +β-brass has lower ductility and greater hardness than the structure of α-brass
Depending on the heating temperature, the following structural transformations occur in brass.
- When brass is heated to high temperatures, the atoms in its β-phase, which has a wide region of homogeneity, are characterized by a disordered arrangement. In such a heating state, the β-phase of the brass alloy is highly ductile.
- With slight heating of the brass alloy (454–468°) a phase with the designation β' is formed in it. A feature of such a structural phase, which is characterized by high hardness and, accordingly, brittleness, is that the copper and zinc atoms in it are arranged in an orderly manner.
The ductility of brasses with a two-phase structure can be increased by heating them above the temperature at which the β'-transformation occurs (700°). In this state, only one β-phase prevails in the structure of the alloy; accordingly, it is characterized by high plasticity. However, even single-phase brasses with good ductility can hardly be processed by plastic deformation methods. This occurs in the temperature range of their heating up to 300–700°, which is called the zone of fragility.
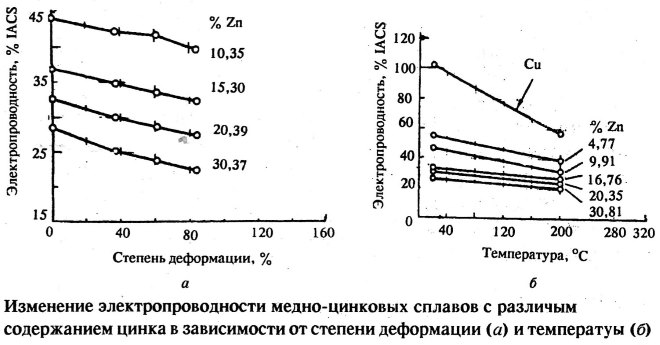
The zinc content in brass affects the electrical conductivity of the alloy
The mechanical properties of brass of a particular brand are significantly affected by the zinc content in its chemical composition. So, if the content of this chemical element is up to 30%, then both the strength and ductility of the alloy increase simultaneously. A further increase in the zinc content leads to the fact that brass becomes less ductile (complication of the α-phase), and then more brittle (formation of the β'-phase in the brass structure). The strength of brass increases until the moment when zinc in its composition is 45%, with a further increase in the amount of this element, brass becomes both less durable and less ductile.
Best Answers
Vladimir Chudentsov:
In fact, faucets are not made of chrome. They are only covered with a thin layer of chromium. And the mixers themselves are made of brass (alloy) or copper (now very rare). So if you are told that the mixer is entirely made of chrome, then most likely you are being deceived. It would be fragile and obscenely expensive.
SEVER velloris:
if exactly then from ls59 chrome only coating
Evgeny Levichev:
A normal manufacturer is made of brass without using the powder method (there is one) And especially cunning narrow-eyed manufacturers from silumin (and for weight they manage to shove steel in there to weigh like brass) and of course chrome is a must as a coating.
bronze alloy price
Modern metallurgical plants and enterprises increasingly prefer to use the processing of recyclable materials to further obtain bronze from it, rather than mining directly from mines. This is due to greater economic efficiency. It is cheaper to melt down existing bronze than to develop new deposits. This is the reason for the constant opening of scrap metal collection points. It remains to understand how prices for bronze scrap are formed.
There are several criteria by which the cost of a bronze alloy is formed:
- Chemical composition. The more the alloy contains scarce and, accordingly, expensive metals in its composition, the higher the price of bronze. These include copper, tin, beryllium. The content of aluminum, silicon and zinc, on the contrary, leads to a reduction in the price of bronze.
- Scrap shape, i.e. in what form bronze is supplied: mesh, wire, shavings, sheet.
- Impurity content and appearance. The presence on the surface of bronze alloys of tin and impurities such as sulfur, hydrogen, phosphorus (over 0.5%) negatively affects the cost of scrap.
- Scope of delivery. In general, the metal receiver prefers working with scrap weighing from 1 ton. Therefore, the larger the mass of the party, the higher the price of bronze.
- Location of the reception point. Different regions have different prices for bronze. This is due to the ratio of supply and demand in a particular region of Russia.
All of the above is taken into account in the state standard GOST 1639-93. According to it, the scrap of bronze alloys is divided into the following categories (its estimated cost per kg is indicated in brackets):
- Scrap A-11-1. Represented by bronze in the form of pieces no larger than 5x5 cm. Blockage in the alloy up to 3%. (250 rubles)
- Scrap A-11-2. The content of copper alloys is not less than 80%. It is supplied in pieces no larger than 5x5 cm. In the compositions of this recycled bronze, the admixture should not exceed 7%. (230 rubles)
- Scrap A-11-3. includes at least 70% copper, and clogging in the alloy is not more than 7%. (200 rubles)
Such a high cost of the alloy is associated with the depletion of copper ores. According to various experts, they should completely end in 80-100 years. The depletion of copper ores, in turn, leads to an increase in the cost of technologies and drilling equipment. The deeper the ore, the harder it is to "get" it from there.
The proof of all of the above is the importance of quotations on non-ferrous metal exchanges, which have been constantly growing since mid-2015.
Physical Properties
Bronze is an alloy of copper with metals such as tin, aluminum, silicon, etc. The exceptions are brass (copper-zinc alloy) and cupronickel (copper-nickel alloy).
The metal is not amenable to heat treatment (except for beryllium). Mechanical properties are completely determined by the chemical composition and structure. Bronze alloys have lower elasticity (9000-12000 kg/mm2) compared to steel.
The value of the coefficient of friction for almost all bronzes is the same. Density fluctuates within 7500-9100 kg/m3. Melting point 880-1060 ºС. Bronze does not transmit heat well. Its thermal conductivity coefficient is 0.1-0.2 cal/cms. The electrical conductivity is significantly inferior to copper. The value of the specific electrical resistance of the bronze alloy is 0.1-0.17 μΩ*m.
The bronze alloy forms a patina on its surface.It is she who protects bronze from further destruction and reduces the rate of corrosion to 0.0015 mm per year.
Rating: /5 -
votes
Big Encyclopedia of Oil and Gas
Nickel brass
Nickel brass has increased mechanical (sv up to 785 MPa) and corrosion properties, it is processed by pressure in a cold and hot state. Brass LN65 - 5 is used for the manufacture of manometric and condenser tubes, various types of rolled products.
Nickel brass is brass containing nickel as an alloying component.
Nickel brass has good corrosion resistance, improved mechanical properties and abrasion resistance, and is well processed by pressure in hot and cold states. Nickel brass is used for the manufacture of condenser tubes for marine vessels, gauge tubes, paper machine meshes and other products. Under the influence of nickel, brasses increase their corrosion resistance in atmospheric conditions, sea water and under conditions of bacteriological corrosion, and the tendency to corrosion cracking sharply decreases.
Nickel brass is brass containing nickel as an alloying component.
The corrosion resistance of nickel brass can be improved by first passivating them by immersion in 50% nitric acid.
Nickel (see Nickel brass) increases the corrosion resistance of brass in atm. Standard brass LN65 - 5 is produced, which is characterized by high corrosion resistance and increased mechanical strength. Sheets, strips, tapes, pipes, rods and profiles are made from brass LN65 - 5. It is used for condenser pipes, mano-metric.
Nickel (see Nickel brass) increases the corrosion resistance of brass in atm. Standard brass LN65 - 5 is produced, which is characterized by high corrosion resistance and increased mechanical strength. Sheets, strips, mites, pipes, rods and profiles are made from brass LN65 - 5. It is used for condenser pipes, mapo-metric.
In chemical engineering, nickel brass is also used, containing up to 12 - 14% nickel, 26 - 30% zinc and 56 - 62% copper. Egi brass have increased corrosion resistance in alkaline salt solutions, sea water and acids that do not have oxidizing properties. The corrosion resistance of nickel brass can be improved by briefly treating them in a 50% nitric acid solution.
Of the special brasses, nickel brasses should be noted, having the composition: 12 - 14% Ni, 26 - 30% Zn and 56 - 62% Cu.
Of the special brasses, nickel brasses should be noted, having the composition: 12 - 14% Ni, 26 - 30% Zn and 56 - 62% Cu.
LMts 58 - 2, nickel brass type LN65 - 5, tin brass type LO60 - 1, beryllium bronze type Br.
Of the special brasses that have found application in chemical engineering, nickel brasses should be noted, having the composition: 12 - 14% Ni, 26 - 30% Zn and 56 - 62% Cu. These brasses belong to ternary a-solutions, they have high resistance to corrosion in solutions of salts, alkalis and are much more resistant than bronzes in acids that are not oxidizing agents.
A Aluminum, Brass, Silicon Brass, Manganese Brass, Marine Brass, Nickel Brass, Lead Brass, Munz Metal. Semi-finished products are made from them in the form of sheets, mites, strips, pipes, rods and wire.
Brass or nickel-plated coasters
For lovers of traditional tea drinking and collectors, a cup holder is of great importance. This attribute has become a wide field for the activities of folk craftsmen and a kind of historical carrier of alternating eras. Starting from pre-revolutionary Russia and up to the present day, one can build a whole chronicle of significant events reflected in chasing, engraving and casting on coasters of different times.
Coasters with a handle for a glass of precious metals were made mainly to order and belonged to noble dynasties.More affordable products were brass coasters, which appeared earlier than others. Durable and plastic material, resistant to corrosion, in the hands of experienced craftsmen turned into a real work of art. Somewhat later, the development of technology made it possible to produce stainless steel coasters with a protective and at the same time decorative nickel coating. Nickel-plated coasters symbolized the Soviet era, although brass has not ceased to be a sought-after material and has also been embodied in propaganda forms.
Brass or nickel-plated coasters - which is better?
Brass and brass coasters
At that time, brass was obtained by fusing copper and zinc ore, given that zinc was discovered only in the 16th century. ad. The first brass contained a lot of third-party impurities, but its strength and external similarity to gold aroused interest. In 116-117, during the time of Augustus, the Romans used the alloy, minting coins from it. For its golden sparkle, the metal was named orichalcum, which literally meant golden copper.
The metal that we are used to seeing was obtained only in 1781 by the British scientist James Emerson. Now more than 60 grades are produced and used in the industry, each of which differs in the composition of alloying substances and properties.
Brass L 63 enjoys the greatest artistic value. Along with high plasticity, susceptibility to cold working by pressure, rolling and embossing, this brand is perfectly polished, acquiring a luster visually indistinguishable from gold.
The only drawback is the gradual oxidation of the surface, so over time the material darkens and acquires a greenish tint. But the original appearance of a brass product is easily returned by re-polishing.
Brass coasters have a wider historical range. In addition, you can buy a rare brass cup holder belonging to an earlier period in various themes and shapes.
Nickel and nickel-plated cup holders
The first samples of nickel were obtained in 1751 by the Swedish mineralogist Kronstedt. Long before that, when mining copper, Saxon miners often encountered ore similar to copper, but all attempts to smelt metal from it failed. For a long time, red nickel pyrite was used only for coloring glass by local glassmakers.
The properties of nickel resemble iron, but its plasticity and white silvery color immediately attracted attention. In addition, due to the tendency of the metal to natural passivation, the luster was preserved and did not darken over time.
The metal also turned out to be malleable to polishing, acquiring a mirror surface, which added artistic value to it. Today, nickel is widely used in many modern industries. The element is most in demand as an alloying component in the production of stainless steels. For plating other metals (nickel plating), approximately 7% of the nickel produced is used.
Nickel-plated coasters have a steel base, so they are more practical and durable. Moreover, the appearance of the products is almost timeless. Perhaps nickel-plated coasters are not as old-fashioned as brass ones. However, these tea attributes have a high historical value and have an exceptional ability to return for a moment to a time when dreams seemed to be a reality, and all roads were open.
Expert answers
Roman Butenko:
copper bronze
Wild_Thing:
The samovar can be made of stainless steel, copper or brass.
Andrey and Lyudmila Vershinin:
I got copper.
Nikitinsky:
samovars used to be made of brass...
Dmitry Solodkov:
the same as the copper basin
zzxx:
Yes, they are copper or brass. Just don't let that fool anyone! Remember old Soviet films? ! “Puddle-u-u-u, pay-yu-yu. » THEY WERE ALL TINNED! In my time…
Luda Vakulko:
brass
Vasya:
They used to be made of copper, but now they are made of stainless steel.
Angela:
How can you clean an old brass samovar
To remove blackness and greenery, the surface of a copper or brass product is wiped with a swab dipped in ammonia (an aqueous solution of ammonia), and then the metal is rinsed with warm soapy water. Ammonia NH3 reacts with copper compounds, which give a black and green coating, to form a highly water-soluble ammonia complex.
Another copper and brass surface cleaner is a mixture of oxalic acid (1 g), ethyl alcohol (5 ml), turpentine (4 ml) and water (1 ml). It is shaken and applied to the area to be cleaned with a soft cloth swab, and after 5-10 minutes the product is wiped with a dry cloth. Turpentine and alcohol degrease the surface, and oxalic acid interacts with copper compounds to form a salt - copper oxalate CuC2O4, which is easily removed from the metal surface.
An old cleaner for copper and brass objects is "vinegar dough". This is a mixture of flour and table vinegar, which is kneaded immediately before use. The "dough" is applied to a metal surface, allowed to dry, and brushed off with a brush or cloth. Acetic acid reacts with copper oxide and copper hydroxide-carbonate in the same way as oxalic acid, only as a result, not oxalate, but copper acetate Cu(CH3COO)2 is formed. Flour also contributes to the cleaning of the product: it serves as an adsorbent for contaminants.

"Metal Polish" manufactured by AUTOSOL Germany is a super polish for samovars. It replaces the mechanical polishing of samovars. Polish for all metal parts: chrome, brass, copper, nickel, aluminium, stainless steel. The polish easily cleans the surface from micro burrs, chemical streaks, surface rust, welding marks, leaving a clean shine protected by an anti-corrosion microfilm. magazin-samovarov /about_samovars/covet cpechialicta/sovetupoyxody/ .raskopki39 /f/viewtopic.php?f=15&t=244 otvet.mail /question/6321170
Misha Walk:
once upon a time, such things were cleaned well with “sourzha” paste — I don’t know whether they sell it now or not .... or do it yourself - thin shaping with ammonia ... like asidol, but shaping stronger
Conclusion
People involved in the collection, delivery and acceptance of non-ferrous scrap need to know and be able to distinguish outwardly similar non-ferrous metals. The ability to identify can pay off well, as brass is nearly half the price of first grade copper at the point of sale.
If the found object is small, you can determine it yourself. If the amount of scrap is large, you can resort to the help of tools or an analyzer that is rented.
If you decide to hand over non-ferrous scrap metal, then make sure that the collection point has a license for this.
How to clean these non-ferrous metals before delivery, you can see in this video: