The main properties of caustic soda
Due to the fact that it quickly dissolves in water, such an alkali has good properties to absorb vapors from the air. Such an alkali has a number of characteristic properties:
- soluble in water and alcohol;
- has a high melting point;
- actively reacts with some metals;
- neutralizes grease and other contaminants.
Caustic soda is widely used precisely because of its main property - degreasing surfaces and neutralizing other contaminants. This ability allows you to get rid of blockages and dirt, which is very helpful when cleaning sewers in private and apartment buildings.

,
ÑоÐ'Ñ ÐμÐ'кий нР° ÑÑ ÑÑÐ ° нÑпоÑÑиÑÑÑÑ Ð¶ÐμÐ »ÐμÐ · ноÐ'оÑожнÑм, воÐ'нÑм, Ð ° вÑомоР± ил ÑнÑм ÑÑÐ ° нÑпоÑÑом в кÑÑÑÑÑ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐ · п Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° гÑÑзов, данном на дейÑÑвÑÑÑими виде ÑÑанÑпÑоои
Ð Ð Ð ÐμÐ'Ð'ÐðкР°ÐðкÐðÐñÐ °Ð РРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРкððñññμμððððððñññ¸ññññ¾ñ ððñññ Ð Ð Ð Ð Ð Ð Ð Ð Ð ñо Ð Ð Ð Ð Ð ñÐñð °¾¼¼ð¼. Ð Ð ° ÑÑÐ²Ð¾Ñ ÑÐμÑниÑÐμÑкого ÐμÐ'кого ÑÑÐ ° нÑÑ Ð½Ð ° ÑÑÐ ° в Ð · Ð ° кÑÑÑÑÑ ÐμмкоÑÑÑÑ Ð¸Ð · мР° ÑÐμÑиР° Ð »Ð °, ÑÑойкого к СпÐμÑиР° Ð »Ð¸Ð·Ð¸ÑованнÑе. Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · Ð¶Ð½Ñ Ð¿ÑомÑÑÑ.
оððÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð · гоÑовР»ÐμннÑми иР· киÑл оÑоÑÐμÐ »Ð¾ÑÐμÑÑойкой ÑÐμÐ · Ð¸Ð½Ñ ÑвÐμÑÐ'оÑÑи ÑÑÐμÐ'нÐμй по ÐÐСР¢ 7338.
Ð ÐμÐ'Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÑÐºÐ¸Ñ Ð² ÑежимммеÑениÑÑ
Ð Ð ÐμÐÐ'Ð'ппповпппРРРРРРРРРРРРÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ , полиÑÑиленовÑм п¿ Ð Ð Ð Ð Ð Ð £ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð μ ROOM 3 ROOM.
Ð Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ Ð Ð Ðμ Ð Ðμð½¸ññ
Precautionary measures
Processing of livestock pens, chicken coops and veterinary offices is carried out manually
Therefore, it is required to observe the precautions for the safe use of solutions:
Be sure to wear protective equipment - goggles, a mask, rubber gloves, swamp clothes or a special suit
It is important to avoid exposed skin surfaces that can get liquid and cause burns. You can not immediately use the prepared composition - soda gives a reaction when mixed with water
Therefore, they wait for its completion and only then start work. You can not spray the product - the surfaces are treated with hands to prevent liquid from getting on the enamelled elements. If the liquid has got on the skin, the affected area is treated with 2% boric acid. Within half an hour, burning and redness should pass. If there is no improvement, consult a doctor for help. It is important to properly store the caustic - it is placed in an iron or glass jar. The container is closed tightly with a lid.
Expert opinion
Carefully!
It is impossible to allow the storage of a caustic solution in free access for children or explosive and fire hazardous equipment.
Size: 39, 997
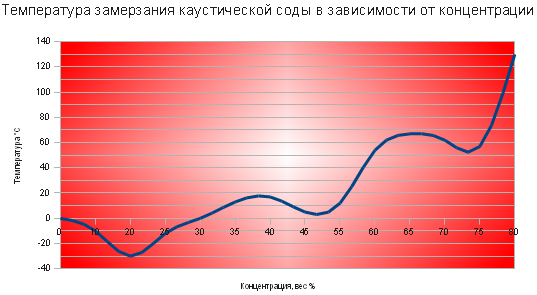
44% 140-142°С 140-142°С
Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° РРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРей Ñавна 44% +7°С. Ð Ð ²ÐðððмÐñооÐμÐμоР°ÐμÐμÐðÐ °Ð °ÐμÐμºñ¸¸¸¸ðμμºñÐ °Ð²¾¾'½½½ Ð Ð Ð Ðμ1Ð Ð Ð Ðμ one:
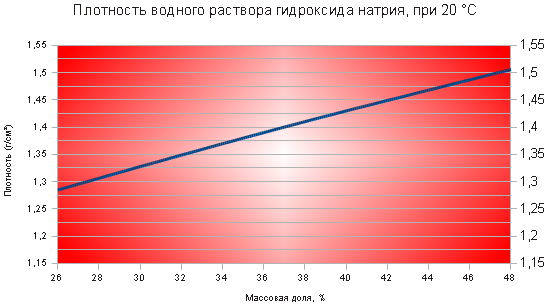
ÐлоÑноÑÑÑ 44%-го ÑаÑÑвоÑа in 1, Ñм3 г/468 . Ð ²Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð ÐμÐ Ð Ð Ð ÐμÐ Ð Ð Ð ÐμÐ Ð Ð ÐμÐñ Ð Ð Ð ÐμÐñ
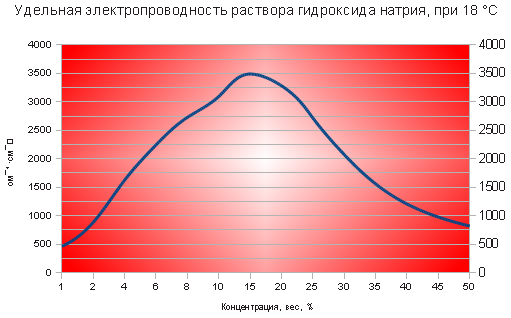
Ð Ð Ð ññ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐðññ¾¾ðððð¾ð¾ððððððððððððð УделÑÐ½Ð°Ñ ÑлекÑÑопÑоводноÑÑÑпÑедÑÑавленѰ на »
Features of working with alkali
Possible consequences
In case of careless handling:
- alkali can get on the skin or mucous membranes and cause severe burns;
- dangerous contact with the eyes, it is fraught with serious consequences in the form of atrophy of the optic nerve;
- inhalation of vapors will lead to damage to the mucous membrane of the larynx and esophagus and may even be fatal.
Recommendations for working with caustic soda
When using caustic soda, precautions are necessary:
- you can’t take the crystals of the substance with your hands, you should have a scoop or spoon for these purposes;
- it is necessary to use special clothes and shoes, rubber gloves and goggles;
- when alkali comes into contact with the skin, it is imperative to wash this area with a large amount of H2O, preferably at room temperature;
- you can treat the burn with a weak solution of citric or acetic acid;
- in case of contact with the mucous membrane, it is necessary to rinse the wound with water and treat with a 2% solution of boric acid.
Storage requirements
Storing caustic soda also requires caution:
- due to its high hygroscopicity, it is packed in sealed bags;
- caustic should be stored in an unheated room;
- if part of the soda solution remains unused, it must be poured into a special alkali-resistant container;
- close it tightly and place in a cool and dry room;
- soda should be kept out of the reach of children;
- after working with it, it is necessary to thoroughly clean the entire area from traces of alkali.
Purification technology
Sewer pipes gradually become dirty and require regular cleaning. Dirt, grease and other organic compounds accumulate on the inner surface of the pipeline. From time to time, pipes become clogged, causing trouble for residents of residential buildings. Previously, mechanical methods were used to clean the sewers, today new methods have appeared. However, it is better to prevent blockage than to deal with it later. Sodium hydroxide is an effective means of preventing blockages in the sewer. Various schemes for cleaning sewers with caustic soda are used.
Use in liquid form
To clean the pipes with soda, the finished alkali solution is poured into the sewer. Within an hour, the drain into the sewer drain stops. At this time, the reaction of decomposition of organic substances accumulated in the pipe takes place. After an hour, it is washed with plenty of hot water.
Caustic soda powder or solution can be replaced with a gel. It also effectively removes dirt. To clean the sewer you will need a glass of funds. The gel can be used once every two weeks for preventive treatment of sewage.

Dry matter application
Carefully pour three tablespoons of baking soda into the drain hole of the bathtub or sink and pour two cups of hot water into it. After 10 minutes, add the same amount
The drain must not be used for about an hour. Then about 10-15 liters of H2O should be poured into the pipe.
 It is necessary to take caustic and acetic or citric acid in equal parts and pour it into the sewer. The hole should be closed, as a violent reaction will begin with the release of abundant foam. After two hours, the pipe must be washed with a strong jet of hot water.
It is necessary to take caustic and acetic or citric acid in equal parts and pour it into the sewer. The hole should be closed, as a violent reaction will begin with the release of abundant foam. After two hours, the pipe must be washed with a strong jet of hot water.
Caustic soda is also effectively used in the fight against contamination of industrial boilers and pipes. A high degree of cleaning is achieved by blowing pipes using this tool. The process takes place at temperatures above 150 degrees for almost 20 hours.
Soda treatment is also required for the hydrodynamic method of cleaning sewer pipes. They are blown by a powerful jet of water, which is supplied under pressure. After removing loose accumulations in the pipes, they are treated with a solution of caustic soda. Caustic soda is excellent for clearing drain blockages if used regularly and in accordance with safety regulations.
Related video: Caustic soda
https://youtube.com/watch?v=11SzA5HWjkU
A selection of questions
- Mikhail, Lipetsk — What discs for metal cutting should be used?
- Ivan, Moscow — What is the GOST of metal-rolled sheet steel?
- Maksim, Tver — What are the best racks for storing rolled metal products?
- Vladimir, Novosibirsk — What does ultrasonic processing of metals mean without the use of abrasive substances?
- Valery, Moscow — How to forge a knife from a bearing with your own hands?
- Stanislav, Voronezh — What equipment is used for the production of galvanized steel air ducts?
Blockage Cleaning Technique
In order to clean the pipe in which the blockage has formed, you must first shake the container in which the soda is stored well.
Important: caustic can be supplied in liquid form, as well as in the form of granules. A little substance (2-3 tablespoons) is poured or poured out (depending on the consistency) into the pipe drain
Then a glass of water is poured into it.

The water must be hot. There is a break of two hours. After it, a powerful jet of water is directed into the drain. When it is required to flush the pipeline, this substance is also used. Here, shock wave technology is used, directing, under pressure, water with caustic soda into the pipe. This allows you to remove scale and rust from the inner surface of the pipes.
Benefits of using caustic soda
Despite the high chemical activity, caustic soda is included in almost all products recommended for cleaning sewers. For domestic needs, it is available in the form of gels, powder or granules. The method of cleaning sewers with sodium hydroxide is convenient because:
- the reagent perfectly copes with various types of pollution in the sewer;
- alkali has a corrosive effect on fatty deposits and other organic compounds that accumulate in sewage;
- neutralization with alkali is necessary after cleaning the sewer with acid, which has a corrosive effect on pipes;
- caustic soda also destroys solid contaminants in the sewer, softening and decomposing them, after which they are easily washed out of the pipeline;
- The advantages of the application include also the availability of the product and ease of use.
Some disadvantages of this method are associated with the chemical activity of caustic soda:
- when cleaning bathtubs or sinks, an aggressive alkaline environment destroys enamel over time;
- protective equipment is required during work.
Exclusive soap
Many housewives prefer to use self-made soap. It is safer, as high-quality and proven components are used.
Sequencing:
Vegetable oil - 500 ml is poured into an enamel saucepan and slightly heated. In a separate earthenware dish, 70 g of caustic soda and 150 ml of distilled water are combined. After the end of the violent reaction, the soda solution is poured into the oil.Stir with a wooden spatula until the caustic is completely dissolved. After obtaining a fairly thick homogeneous mass, aromatic herbs ground into powder are introduced - 2 tbsp. l. or essential oil with your favorite scent - 5 drops. Pour the gel-like substance into a low container, preferably rectangular. Put in a dry place
Four days later, with a sharp knife, the resulting soap is carefully cut, forming pieces of the desired size, and left to ripen.
Expert opinion
Recommended!
The readiness of the soap is determined by the whitish coating that appears on the surface. The process takes up to one and a half months. A homemade soap with impressive cleansing qualities lathers well and has an undeniably pleasant scent.
Applications of caustic soda
Due to its high reactivity, caustic is widely used in many industries and in various areas of human activity:
- in chemical production, where it acts as a catalyst for the synthesis of complex organic fibers;
- in obtaining pure metals;
- for processing pulp in the papermaking process;
- soap making and production of detergents and cleaners;
- pharmaceutical and food industry;
- oil refining industry;
- cosmetic industry;
- production of textiles, glass and many other goods.
World production of caustic soda reaches 57 million tons per year. In everyday life, caustic soda is most often used:
- to remove blockages in the sewer;
- cleaning the drain holes of bathtubs and sinks;
- in home soap making.
More information about the tool
Where to buy caustic soda
Caustic soda is a consumer product. You can buy it in the department of the hardware store that sells equipment and supplies for gardeners, or in retail outlets with building materials.
Working with caustic soda at home is not very convenient, especially if there are small children and animals. Despite this, the tradition of use in everyday life is preserved, which can be explained by the effectiveness and low cost of the product. When using alkali, be careful, then the caustic will become your reliable assistant in maintaining cleanliness.
When working with caustic soda, take precautions - rubber gloves are a must
Safety regulations
Alkali has properties that require caution. When working with a caustic substance, follow the rules to avoid caustic soda poisoning and burns:
Wear rubber gloves, goggles, clothing that covers the skin.
Before starting work, check if the caustic will come into contact with metals
Such a "meeting" can provoke an aggressive reaction.
Follow the recommended proportions of water and caustic soda, this is especially important when cleaning enamelware and soaking fabrics.
Wash your hands well after handling the substance.
Try to work with caustic soda in a room with open windows, close the container with soda - the maximum allowable concentration in the air is 0.5 mg / m³.
Caustic soda should be stored in closed containers and in a place that is guaranteed to be protected from children and pets.
GOST 2263 regulates the transportation, storage and packaging, shelf life of caustic soda. Liquid or granular sodium hydroxide must be in a container that does not react with the substance and ensures tightness
Store the substance at home in its original packaging.
What to do with a caustic burn
NaOH burns are dangerous and not uncommon. When concentrated alkali gets into open areas of the body, the substance penetrates deep under the skin and can cause tissue necrosis. Contact with eyes may result in loss of vision.
First aid for burns of the mucous membrane or skin with caustic - immediate washing in a large amount of running water and treating the affected area with boric acid.If lye gets into your eyes, you should immediately rinse them under running cold water and consult a doctor as soon as possible.
Precautions for handling sodium hydroxide
Chemical burn due to sodium hydroxide solution. Photo taken 44 hours after exposure
Sodium hydroxide is caustic, toxic and corrosive. It belongs to substances of the second hazard class.
Therefore, care must be taken when working with it. Contact with skin, mucous membranes and eyes causes severe chemical burns
Contact with the eyes causes irreversible changes in the optic nerve (atrophy) and, consequently, loss of vision. In case of contact of mucous surfaces with caustic alkali, it is necessary to wash the affected area with a stream of water, and in case of contact with the skin, with a weak solution of acetic or boric acid. If caustic soda gets into the eyes, immediately rinse them first with a weak solution of boric acid, and then with water.
When working with caustic soda, the following protective equipment is recommended: chemical splash goggles for eye protection, rubber gloves or gloves with a rubberized surface for hand protection, and chemical-resistant vinyl impregnated clothing or rubberized suits for body protection.
The maximum allowable concentration of sodium hydroxide in the air is 0.5 mg/m³.
Physical Properties
Sodium hydroxide is a white solid. It is highly hygroscopic, “spreads out” in air, actively absorbing water vapor from the air. It dissolves well in water, while a large amount of heat is released. A solution of caustic soda soap to the touch.
Thermodynamics of solutions
ΔH dissolution for an infinitely dilute aqueous solution −44.45 kJ/mol.
From aqueous solutions at +12.3 ... +61.8 ° C monohydrate crystallizes (rhombic syngony), melting point +65.1 ° C; density 1.829 g/cm³; ΔHarr -425.6 kJ / mol), in the range from -28 to -24 ° C - heptahydrate, from -24 to -17.7 ° C - pentahydrate, from -17.7 to -5.4 ° C - tetrahydrate ( α modification). Solubility in methanol 23.6 g/l (t = +28 °C), in ethanol 14.7 g/l (t = +28 °C). NaOH 3.5H2O (melting point +15.5 °C).
Sewer blockages
The properties of caustic soda are widely used in various types of industry. Mineral fertilizers are made from it, used to clean sewers. With the problem of clogging sewer pipes, caustic soda becomes an effective tool for cleaning them. There are several options for cleaning sewers:
- Pour 4 tablespoons of caustic soda into the pipe hole, then pour 300 ml of boiling water. When 2 hours have passed, rinse with plenty of water.
- Vinegar and soda - 1: 1. Pour 130 grams of each substance into the sewer until foam begins to form. After that, it is better to close the pipe hole with a cork for 2 hours. After the required time, rinse with boiling water.
- Mix 7 liters of water and 4 kilograms of caustic. Pour everything into the sewer pipe - such a liquid mixture is a very effective option for flushing pipes.
Caustic soda is very often used to clean drains, it is the best option for removing blockages in pipes of various kinds.
The use of caustic for sewerage cleaning is especially effective in multi-storey buildings, apartments, where it is very problematic to restore the patency of the pipe in a different way.
This alkaline substance is also used for other household purposes. A liquid solution of caustic soda will clean not only the sewers, but also old coins that have lost their appearance due to long-term storage.
Application in everyday life
However, in addition to this, caustic soda is quite often used in the manufacture of soap. With the help of caustic soda, you can solve at least 8 household tasks:
- Get rid of scale in the kettle.
- Clean the soot on the dishes.
- Make soap, use as dishwashing detergent.
- Clear the blockage in the sewer.
- When washing instead of powder.
- As a bleach and stain remover.
- As a floor cleaner.
- Cleaner for sanitary ware.
Caustic soda can be used as a detergent. For example, instead of washing powder. It can also be used to clean floors. Recipes for the most common uses are discussed below.

What is caustic soda
Caustic soda, sodium hydroxide, caustic are the names of the same alkali. Caustic soda, the chemical formula of which is NaOH, unlike sodium carbonate (soda ash) and potassium carbonate (potash) is very active - a high concentration causes burns, corrodes substances of organic origin. One of the strongest alkalis is a white solid with no pronounced odor. NaOH dissolves easily in water and absorbs moisture from the air with the release of a large amount of heat. Alkali is transported in waterproof bags or in the form of a solution.
Caustic soda has a set of specific properties:
- Easily soluble in water and alcohol solutions, but insoluble in ethers and acetone.
- The substance is non-combustible, with a very high melting and boiling point (1390°C).
- Actively reacts with aluminum, lead, zinc and tin.
- Forms explosive gas, ignites in reaction with ammonia.
- Molten caustic soda is destructive to glass.
- Neutralizes fats and organic impurities.
Industrial production of caustic soda began in the middle of the 18th century, then ammonia and lime methods were used, the second was more common until the 20th century. At the turn of the XIX-XX centuries. invented an electrochemical method by which most of the caustic is now obtained. The leaders in production are now China, the USA and the EU countries. The output of sodium hydroxide lags behind world indicators, but it satisfies the needs of the domestic market.
Characteristic properties of caustic soda
Caustic soda or caustic soda is most often a powder in the form of tiny crystals or solid white granules, odorless. Due to the high hygroscopicity in air, they quickly blur, absorbing moisture. The substance is excellently soluble in H2O, forming a soapy liquid to the touch and releasing a large amount of heat: 46 g of soda dissolves in 100 g at a temperature of 20 degrees Celsius.

The solid has a melting point of just over 65 degrees Celsius. When dissolved, the molecules of a chemical compound pass into a hydrated form with a lower melting point - 15.5 degrees. Caustic soda dissolves well in methyl and ethyl alcohols.
The name itself - Caustic Soda or caustic soda, indicates the aggressive nature of this substance. Natural soda was known to the ancient Egyptians and Greeks, who used it in the manufacture of paints, embalming, bleaching canvases.
Aqueous solutions of caustic soda have a strong alkaline reaction - the pH of a 1% solution is 13. Their storage and transportation requires special safety conditions - special steel containers. Therefore, most often sodium hydroxide is produced in solid form and transported in sealed bags.
2263
NaOH
Сð¾Ð ° кÐ𠸸ðμμðººð °'¸ðð)) )ºº μμ¸ðððð¾¾¾¾¾¸μμμμð¾ð¾¾¸¸¼½½ððμºº¾¸¼¼¼μ½ºº² ²ð¸²ð½²² Ð1ð²²μμμ
NaOH pH pH 1%-3% pH.
ÑÐ ° ñ ñ ²Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð μ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ðμ ж Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð
Ð Ð Ð Ð ÐμÐ Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² в ÐμÑ ÑÐμÐ · ÑÐ »ÑÑÐ ° ÑÐμ, в Ðμго ÑÐμго, Ñо вÑÐμмÐμнÐμм, оР± ÑÐ ° Ð · ÑÐμÑÑÑ Ð ± Ðμл Ñй ÑоÐ'Ð ° - оÑÐ ° Ð'ок кР° Ð »ÑÑиниÑовР° ннР° Ñ (Na2CO3)
Ð Ð ÐμÐðÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð ² Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ² ² ² ñ ²Ð Ð Ð Ð °.
Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð · РРРРРРРРРРРРРРРРРРРРРРРи Ð ° ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ° Ð Ð ° Ð ° Ð ° Ð ° Ð ° Ð ° Ð ° Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð δ Ð Ð μm ´.
Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ²Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð Ð Ð Ð ÐμÐ Ð Ð Ð ÐμРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРРмаÑеÑиалами.
What is caustic soda
Caustic soda, sodium hydroxide, caustic are the names of the same alkali. Caustic soda, the chemical formula of which is NaOH, unlike sodium carbonate (soda ash) and potassium carbonate (potash) is very active - a high concentration causes burns, corrodes substances of organic origin. One of the strongest alkalis is a white solid with no pronounced odor. NaOH dissolves easily in water and absorbs moisture from the air with the release of a large amount of heat. Alkali is transported in waterproof bags or in the form of a solution.
Caustic soda has a set of specific properties:
- Easily soluble in water and alcohol solutions, but insoluble in ethers and acetone.
- The substance is non-combustible, with a very high melting and boiling point (1390°C).
- Actively reacts with aluminum, lead, zinc and tin.
- Forms explosive gas, ignites in reaction with ammonia.
- Molten caustic soda is destructive to glass.
- Neutralizes fats and organic impurities.

Industrial production of caustic soda began in the middle of the 18th century, then ammonia and lime methods were used, the second was more common until the 20th century. At the turn of the XIX-XX centuries. invented an electrochemical method by which most of the caustic is now obtained. The leaders in production are now China, the USA and the EU countries. The output of sodium hydroxide lags behind world indicators, but it satisfies the needs of the domestic market.
Features of disinfection
Disinfection in animal husbandry is not only the correct preparation of the solution, but also the observance of the sequence of cleaning the premises.
Disinfection of livestock buildings
Pre-training means:
- Removal of livestock from the premises.
- Use of a caustic soda solution to remove contaminants.
- Thorough cleaning with water and detergents of walls, floors, ceilings, auxiliary elements.
- Final treatment with a soda solution of a certain concentration of all surfaces of the livestock building.
Poultry farming allows the use of a concentration of 2-3% for routine cleaning of the premises and up to 7% in case of detection of spore diseases.
Premises containing livestock require a higher concentration of caustic soda for standard cleaning - at least 4%, and up to 10% if animal diseases are detected - influenza, brucellosis, anthrax.
Disinfection in veterinary medicine
The rooms used for examining animals are cleaned daily with a concentrated solution of caustic soda.Standard cleaning with a concentration of 1% is allowed, and in the case of an examination of an animal with complex infection, 2-3%.
Caustic soda in other areas
Use in veterinary medicine
In veterinary medicine, the bactericidal properties of alkali are used. The substance penetrates to a depth of 10 cm and disinfects the coverings of animal rooms. A 20% solution destroys anthrax spores, a 10% solution destroys tubercle bacillus, and only six minutes are enough to neutralize staphylococci.
When processing with hot solutions, the rooms should be well ventilated. In cowsheds, pigsties, sheepfolds, etc., there is a large concentration of ammonia, which, in combination with soda fumes, becomes a poison.
Caustic soda is traditionally used as a universal disinfectant in a hot 3% solution (70°C) in epidemics of viral and bacterial diseases.
- Disinfection of hives and soil in the apiary allows you to save bees during periods of pestilence.
- To disinfect premises where animals are kept, the floor and walls should be treated with a 4% solution (2 tablespoons / 1 liter of water). At the time of processing, animals must be removed from the barn.
Application in industry
- Caustic soda degreases tools, containers and equipment for production in the food industry. Food additive E524 containing NaOH is added, for example, to ice cream, caramel and drinks.
- For chemical production, caustic is a versatile catalyst used in detergents and oils.
- The use in the defense sector is due to the ability of soda to neutralize carbon dioxide. Alkali is used in breathing apparatus.
- Alkaline batteries and biofuels in mechanical engineering are made using caustic soda.
- The property of NaOH to break down paper has found application in the manufacture of cardboard.
Preparation of caustic soda solution
In order to dilute soda with water, you should follow certain rules:

the container must be resistant to alkali, for example, a thick-walled plastic bucket with a capacity of up to 12 liters is suitable;
to prepare the product, you need to take only cold water;
it is impossible to pour water into alkali, it is necessary to add soda to it, otherwise splashing of the resulting hot alkali is possible;
since a large amount of heat is released during the dissolution process, the reagent must be added in small portions, with gentle stirring;
hot solution emits caustic fumes, so it is better to use a gauze mask;
it is preferable to prepare a caustic solution in the open air, if this is not possible, then a window should be opened so that the room is ventilated;
per 7 liters of H2O is usually taken from 2 to 4 kg soda, depending on the type of residential building and sewerage system.
Cleaning dishes
Given the effective cleansing effect of caustic soda, housewives use it to remove very stubborn dirt from dishes. It must be borne in mind that it is impossible to process aluminum and Teflon kitchen utensils with this agent.
Suitable for cast iron, enameled and steel varieties, which acquire perfect cleanliness.
- Take a large metal container. Pour 10 liters of water.
- Add caustic - 200 g.
- Pour out thin shavings obtained from grinding one bar of laundry soap.
- Stationary glue is introduced (it is also called "liquid glass") - 150 ml.
- Mix thoroughly and immerse the dishes.
- The container is placed on the stove. With slow heating after boiling, stand for two hours.
- Rinse the cleaned utensils thoroughly with cool water.
Expert opinion
Recommended!
Using this method, you can effectively remove greasy old dirt from the plates. But they should be boiled for 10 minutes.
Handmade soap
Caustic soda is able to foam well and wash dirt well.Therefore, it is used in the manufacture of soap, in particular, at home. There are a lot of recipes for making such a homemade product. The washing power of such soap depends on the concentration of caustic soda in it.
Important: in the manufacture of home cosmetics, it is necessary to use only the highest purity caustic soda
So that the soap has the opportunity to moisturize the skin and care for it, special additives are introduced into it. It can be lemon juice, various oils, glycerin, vitamin supplements. Caustic soda can only be used in compliance with all safety rules. Gloves, goggles, special clothing, a respirator are required.