Exploitation

In vanadium flow batteries, both reference electrodes are additionally connected to storage tanks and pumps so that very large volumes of electrolyte can be circulated through the cell. The circulation of liquid electrolyte is somewhat difficult and limits the use of vanadium flow batteries in industries requiring mobility, making them effective in large stationary buildings.
When a vanadium battery is charged, the VO2+ ions in the positively charged reference electrode turn into VO2+ ions when the electrons are detached from the positive battery terminal. Similarly, in the negative reference electrode, electrons convert V3+ ions to V2+. During discharge, this process is reversed, resulting in an open circuit voltage of 1.41 V at 25°C.
Other useful properties of vanadium flow batteries include a very fast response to load changes and an extremely high overload capacity. Research at the University of New South Wales has shown that they can achieve response times of less than half a millisecond at 100% load changes and withstand 400% overload for over 10 seconds. The response time is in most cases limited by the electrical equipment. Vanadium batteries based on sulfuric acid only work at temperatures of 10-40C. If the temperature is below this range, sulfuric acid ions crystallize. Efficiency in reciprocating motion in everyday use remains at the level of 65-75%.
Features of charging and discharging
Charging algorithms directly depend on how the battery is arranged and what type it belongs to. For example, some batteries can safely replenish their capacity from constant voltage sources. Others work only with an adjustable current source that can change parameters depending on the level of charge.
An incorrectly organized charging process can damage the battery. In extreme cases, the battery may ignite or explode. There are smart batteries equipped with voltage monitoring devices. The main parameters that should be taken into account when operating reversible galvanic batteries are:
- Lifespan. Even with proper handling, the number of charge cycles for a battery is limited. Different battery systems do not always wear out for the same reasons. But in general, the battery life is limited primarily by the number of full charge-discharge cycles, and secondly by the design service life without reference to the intensity of use.
- Charge time. The fundamental design of the battery does not imply charging at an arbitrarily high speed: the internal resistance of the galvanic cell will lead to the conversion of excess charging current into heat, which can irreversibly damage the device. From a physical point of view, the charging time is limited by the maximum diffusion rate of the active material through the electrolyte.Simplistically, we can assume that the restoration of full capacity in one hour is a good indicator.
- Discharge depth. Specified as a percentage of the rated power. Describes the usable capacity. For different types of batteries, the recommended operating discharge level may vary. Due to changes in operation or aging, the maximum depth indicator loses its original value.
diffusion process.
Due to the process of diffusion, the alignment of the electrolyte density in the cavity of the battery case and in the pores of the active mass of the plates, the electrode polarization can be maintained in the battery when the external circuit is turned off.
The diffusion rate directly depends on the temperature of the electrolyte, the higher the temperature, the faster the process takes place and can vary greatly in time, from two hours to a day. The presence of two components of the electrode potential in transient conditions led to the division into equilibrium and non-equilibrium EMF of the battery. The equilibrium EMF of the battery is affected by the content and concentration of ions of active substances in the electrolyte, as well as the chemical and physical properties of active substances. The main role in the magnitude of the EMF is played by the density of the electrolyte and the temperature practically does not affect it. The dependence of EMF on density can be expressed by the formula:
The battery emf is not equal to the battery voltage, which depends on the presence or absence of a load on its terminals.
admin25/07/2011
A comment
Name *
Site
This site uses Akismet to fight spam. Find out how your comment data is processed.
« Mechanical tachometer
Battery voltage »
Tags
VAZ, VAZ malfunctions Sensors Ignition Injector Devices Starter Schematics Electric cars Power supply vaz 2110 gazelle gazelle business registrars car repair
Recent Entries
- Sensors in the car: types and purpose
- The world's largest electric car EDumper,
- Laser lights.
- Advantages and disadvantages of halogen lamps
- The device and principle of operation of parking sensors
Archives
Archives Select SEPTEMBER 2019 August 2017 July 2017 June 2017 May 2017 April 2017 March 2017 December 2016 November 2016 October 2016 September 2016 August 2016 July 2016 June 2016 May 2016 April 2016 March 2016 February 2016 November 2015 October 2015 August 2015 July 2015 June 2015 May 2015 January 2015 December 2014 November 2014 October 2014 September 2014 August 2014 July 2014 June 2014 May 2014 April 2014 February 2014 January 2014 December 2013 November 2013 October 2013 August 2013 June 2013 May 2013 Mart September 2012, 2013, 2012, 2012, 2012, 2012, 2012, 2012, 2011, 2012, 2011, 2012, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, 2011, September 2012, 2011, 2011, 2011, 2011, 2011, 2011
Categories
- Accumulator battery
- Video
- Generator
- Sensors
- Diagnostics
- Ignition
- news
- Equipment
- Devices
- Repair
- Spark plug
- Starter
- Scheme
- Devices
- electric cars
- Power supply
We are in social networks
Auto Electrician@ All rights reserved. When copying site materials, you must provide a link to the site.
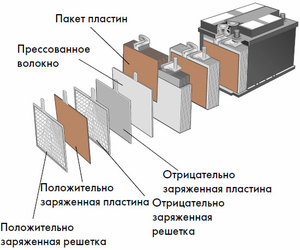
Device and principle of operation
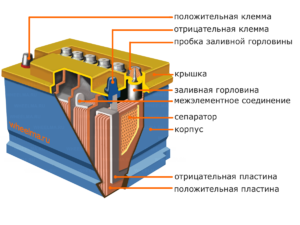
Each such cell has a cathode (positive electrode) and an anode (negative). These electrodes are separated by an electrolyte that ensures the exchange of ions between them. Electrode materials and electrolyte composition are chosen to provide sufficient electromotive force between the battery terminals.
Since the electrodes contain a limited potential of chemical energy, the battery will be depleted during operation. The type of galvanic cells, which is adapted for replenishment after a partial or complete discharge, is called a battery. An assembly of such interconnected cells is a battery.Battery operation involves a cyclical change of two states:
- Charging - the battery works as a receiver of electricity, inside the cells the electrical energy is realized in chemical changes.
- Discharging - the device functions as a source of electrical current by converting the energy of chemical reactions into electrical energy.
Areas of use
The ultra-high capacity characteristic of vanadium redox batteries makes them well-suited for use in industries requiring high energy storage. For example, helping to balance the volume of production of such energy sources such as wind or solar, or helping generators to absorb large surges of energy when needed, or balancing supply and demand for energy for remote areas.
The limited self-discharge characteristics of vanadium redox batteries make them useful in industries where batteries must be stored for long periods of time with minimal maintenance and readiness. This led to their use in some types of military electronics, for example, in the sensors of the GATOR mining system. Their ability to cycle through and stay at zero makes them suitable for solar applications and industries where batteries must start the day empty and recharge based on load and weather. For example, lithium ion batteries are often damaged when they are allowed to discharge below 20% of their volume, so they most often operate in the 20 to 100% range, which means they can only use 20% of their rated capacity.
Their extremely fast response time also makes them practically indispensable for uninterruptible power supplies, where they can be used in place of lead-acid batteries and even diesel generators. Also the fast response time makes them suitable for frequency control. At the moment, neither UPS nor frequency management measures are effective on their own, but it is likely that the battery will be able to find applications in these industries when capitalized from various sources of funding. In addition, these capabilities make vanadium redox batteries an effective "one-piece" solution for small power grids that depend on reliable operation, frequency control, and load switching needs (such as high penetration of renewables, highly fluctuating loads, or the desire to optimize generator efficiency with by shifting the response time).
The largest working vanadium redox batteries
Substation "Minami Hyakita":
- Launch date: December 2015
- Energy: 60 MWh
- Power: 15 MW
- Working time: 4 hours
- Country: Japan
Stinky, Liaoning Province
- Launch Date: N/A
- Energy: 10 MWh
- Power: 5 MW
- Working time: 2 hours
- Country: China
Tomamae Wind Farm
- Launch date: 2005
- Energy: 6 MWh
- Power: 4 MW
- Working time: 1 hour 30 minutes
- Country: Japan
Zhangbei Project
- Launch date 2016
- Energy: 8 MWh
- Power: 2 MW
- Working time: 4 hours.
- Country: China
SnoPUD MESA 2 project
- Launch Date: March 2017
- Energy: 8 MWh
- Power: 2 MW
- Working time: 4 hours.
- Country: USA
Substation in Escondido
- Launch date: 2017
- Energy: 8 MWh
- Power: 2 MW
- Working time: 4 hours.
- Country: USA
Substation in Pullman, Washington
- Launch date: April 2015
- Energy: 4 MWh
- Power: 1 MW
- Working time: 4 hours
- Country: USA
By 2018, the development of a vanadium redox battery is expected to be completed in China. Its energy will be 800 MWh, power - 200 MW, and operation time - 4 hours.
Terms
- Sequential - elements follow one after the other.
- Electromotive force (EMF) is the voltage generated by a battery or magnetic force in accordance with Faraday's law.
- Parallel - Electrical components are arranged so that current flows along two or more paths.
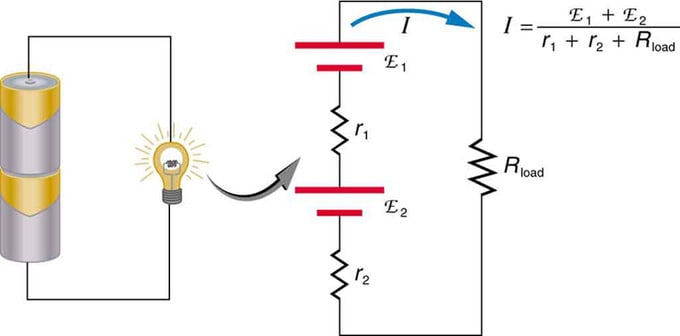
If you are using multiple voltage sources, they can be connected in series or in parallel. With the series version, they are tuned in the same direction, the internal resistance is plused, and the electromotive force is added algebraically. Similar types are common in flashlights, toys, and a variety of other appliances. Cells are placed in series to increase the total emf.
Serial connection of two voltage sources in the same direction. The diagram shows a lantern with two cells and one lamp
Battery - multiple connection of volt elements. But there is one disadvantage in serial connection, as internal resistances are added. Sometimes this creates problems. Let's say you have two 6V batteries that you put in instead of the usual 12V. As a result, you have added not only the EMF, but also the internal resistance from each battery.
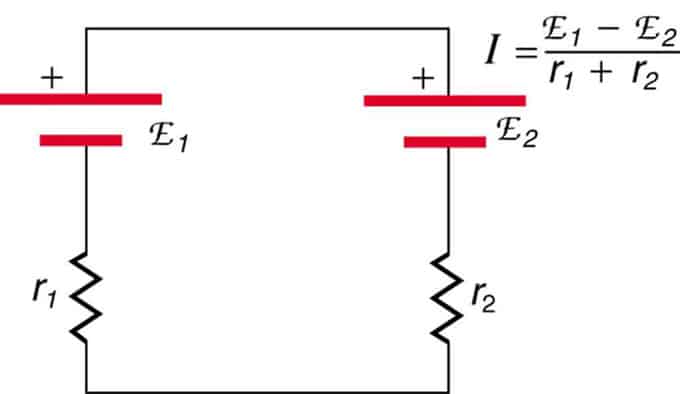
If the cells are located in opposition (one is located behind the other), then the total emf will decrease.
These are two voltage sources connected in series with opposing emissions. The current flows in the direction of greater EMF and is limited by the summation of internal resistances. An example is a charger. It must have more emf than the battery
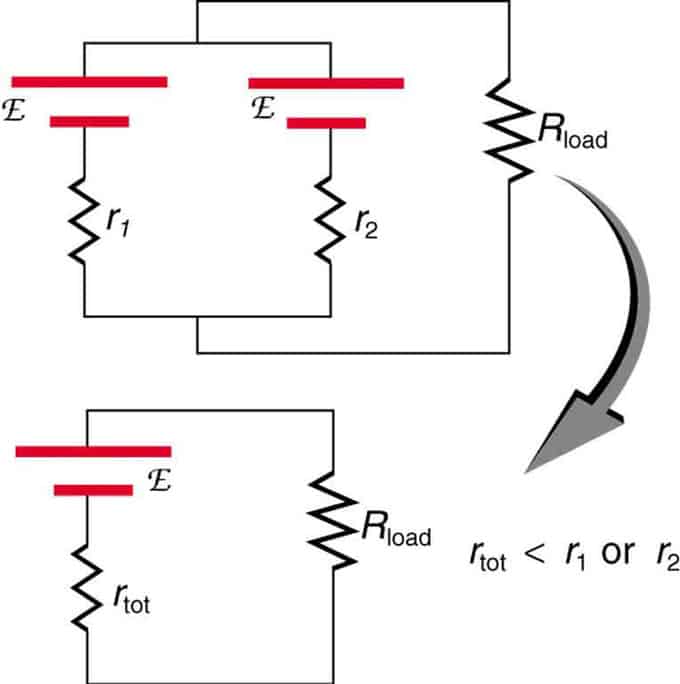
If two sources with the same electromotive force are located in parallel and connected to the load resistance, then the total EMF remains the same as the individual ones. However, the total internal resistance will be reduced. It turns out that the parallel version can generate more current.
Two voltage sources with a single EMF are combined in parallel connection. They form one EMF, but have less total resistance than individually. Similar combinations are used if you need to achieve more current.
| Overview |
|
| Parallel and series connection of resistors |
|
| Kirchhoff rules |
|
| Voltmeters and ammeters |
|
| RC Circuits |
|
Historical overview
The development of the first galvanic cell is credited to the Italian physicist Alessandro Volta. He conducted a series of experiments with electrochemical phenomena during the 1790s and around 1800 he created the first battery, which his contemporaries called the "voltaic column". The device consisted of alternating zinc and silver discs separated by layers of paper or cloth that were soaked in a sodium hydroxide solution.
These experiments became the basis for Michael Faraday's work on the quantitative laws of electrochemistry. He described the principle of operation of the battery and, based on the work of the scientist, the first commercial electrical cells were created. Further evolution looked like this:
- In 1836, the British chemist John Daniel presented an improved model of the cell, consisting of copper and zinc electrodes immersed in hydrochloric acid. Daniel's element was able to provide constant voltage incomparably more efficiently than Volt's devices.
- 1839 Further progress was made by the physicist Grove with his two-fluid cell, consisting of zinc immersed in dilute sulfuric acid in a porous container. The latter separated sulfuric acid from a vessel containing nitric acid with a platinum cathode placed in it. The nitric acid served as an oxidizing agent to prevent voltage loss due to hydrogen accumulation at the cathode.The German chemist Robert Bunsen replaced the platinum with inexpensive carbon in the Grove cell and thereby promoted the widespread acceptance of this type of battery.
- In 1859, Gaston Plante invented the lead-acid cell, the forerunner of the modern car battery. Plante's device was able to produce an unusually large current, but was used only for experiments in laboratories for almost two decades.
- 1895-1905 years. Invention of nickel-cadmium and nickel-iron alkaline elements. This made it possible to create systems with a significant number of charge-discharge cycles.
- Since the 1930s, the development of silver-zinc and mercury-zinc alkaline batteries began, which provided high energy density per unit weight and volume.
- Since the middle of the 20th century, advances in manufacturing technology and the advent of new materials have led to even more powerful and compact batteries. Most notable was the introduction of nickel-metal hydride and lithium batteries to the market.
Charging batteries
Main article: Charger
As the chemical energy is depleted, the voltage and current drop, and the battery ceases to function. You can charge the battery (battery of batteries) from any DC source with a higher voltage while limiting the current. The most common is the charging current (in amperes), proportional to 1/10 of the conditional nominal capacity of the battery (in ampere hours).
However, based on the technical description distributed by manufacturers of widely used electric batteries (NiMH, NiCd), it can be assumed that this charge mode, commonly referred to as standard, is calculated based on the duration of an eight-hour working day, when the battery, discharged at the end of the working day, is connected to the mains charger before the start of a new working day. The use of such a charge mode for these types of batteries with systematic use allows you to maintain a quality-cost balance in the operation of the product. Thus, at the suggestion of the manufacturer, this mode can only be used for nickel-cadmium and nickel-metal hydride batteries.
Many types of batteries have different limitations that must be taken into account during charging and subsequent use, for example, NiMH batteries are sensitive to overcharging, lithium batteries are sensitive to overdischarge, voltage and temperature. NiCd and NiMH batteries have a so-called memory effect, which consists in a decrease in capacity when charging is carried out when the battery is not completely discharged. Also, these types of batteries have a noticeable self-discharge, that is, they gradually lose charge without being connected to the load. To combat this effect, drip recharging can be used.
Battery Charging Methods
Several methods are used to charge batteries; Generally, the charging method depends on the battery type.
- Slow DC charge
Charge with a direct current proportional to 0.1-0.2 of the conditional nominal capacity Q for about 15-7 hours, respectively.
The longest and safest charging method. Suitable for most types of batteries.
- fast charge
Charge with direct current proportional to 1/3 Q for about 3-5 hours.
- Accelerated or "delta-V" charge
A charge with an initial charge current proportional to the nominal nominal capacity of the battery, at which the battery voltage is constantly measured and the charge ends after the battery is fully charged. Charging time is about an hour and a half. The battery can overheat and even destroy it.
- reverse charge
It is performed by alternating long charge pulses with short discharge pulses. The reverse method is most useful for charging NiCd and NiMH batteries, which are characterized by the so-called.n. "memory effect".