CLAIM
1. The method of direct liquefaction of coal, which includes the following steps:
(1) preparing a coal slurry from raw coal and a catalyst;
(2) mixing the coal slurry with hydrogen and pre-treatment of the mixture, followed by its supply to the reaction system for carrying out the liquefaction reaction;
(3) separating the reaction products withdrawn from the reactor in a separator (9, 10) to form a liquid phase and a gas phase, wherein the liquid phase is subjected to fractionation in a distillation column (11) at atmospheric pressure to obtain a product in the form of a diesel fuel fraction and a residual product ;
(4) feeding the residual product obtained in the atmospheric pressure column to a vacuum distillation column (12) for separation into distillate and residue;
(5) mixing the diesel fuel fraction and the distillate to form a mixture, and then feeding the mixture to the forced circulation fluidized bed hydroprocessing reactor (13) to carry out the hydrogenation process;
(6) fractionation of the hydrogenation products into oil products and a hydrogen donor solvent recycled to stage (1).
2. The method according to claim 1, in which stage (1) includes the following operations:
(a) converting the raw coal into coal powder with a given particle size after drying and grinding the raw coal in a pretreatment apparatus; (b) treating the catalyst feedstock (3) and the coal powder in the catalyst preparation apparatus (4) to obtain an ultra-fine coal liquefaction catalyst powder; (c) mixing in the apparatus (5) for preparing a slurry of the coal liquefaction catalyst and coal powder with a hydrogen donor solvent (16) to form a coal slurry.
3. The method of claim 1, wherein the coal liquefaction reaction step includes the following steps:
(a) feeding the coal slurry after mixing it with hydrogen (6) and preheating it into the first fluidized bed reactor (7) with forced circulation to carry out the liquefaction reaction to obtain reaction products leaving the reactor; (b) feeding the reaction products leaving the first fluidized bed reactor (7), after mixing them with hydrogen, to the second fluidized bed reactor (8) with forced circulation to continue the liquefaction reaction, said fluidized bed reactors operating at the following reaction conditions: reaction temperature 430-465°C; reaction pressure 15-19 MPa; the ratio of the amounts of gas and liquid 600-1000 nl/kg; volumetric rate of coal suspension 0.7-1.0 t/m3 h; the degree of addition of the catalyst Fe/dry coal = 0.5-1.0 wt.%.
4. The method according to claim 1, in which stage (3) includes the following operations:
(a) feeding the stream of reaction products into a high temperature separator (9) for separation into a gas phase and a liquid phase, while the temperature in the high temperature separator is maintained at 420°C;
(b) feeding the gas phase from the high temperature separator (9) to the low temperature separator (10) for further separation into gas and liquid, while the temperature in the low temperature separator is maintained at room temperature.
5. The method according to claim 2, in which -FeOOH is used as a liquefaction catalyst, the particles of which have a diameter of 20-30 nm and a length of 100-180 nm, and the catalyst contains sulfur at a molar ratio of S/Fe=2.
6. The method according to claim 1, in which the hydrogenation in stage (5) is carried out under the following conditions: reaction temperature 330-390°C; reaction pressure 10-15 MPa; the ratio of the amounts of gas and liquid 600-1000 nl/kg; space velocity 0.8-2.5 h-1.
7. The method according to claim 1, wherein the recycled hydrogen donor solvent is a hydrogenated liquefied petroleum product with a boiling point in the range of 220-450°C.
8. Process according to claim 1, wherein the residue in the vacuum distillation column (12) has a solids content of 50-55% by weight.
9. The method according to claim 1, wherein the mixture of the diesel fuel fraction leaving the atmospheric pressure column and the distillate from the vacuum column has a boiling point of C 5 in the range of 530°C.
10.The process according to claim 1, wherein the forced circulation fluidized bed hydroprocessing reactor (13) is an internal reactor, wherein a circulation pump is installed near the bottom of the reactor, and the catalyst in the reactor can be replaced during operation.
CLAIM
1. A method for burning coal, including drying it, grinding it to a finely dispersed state, mixing ground coal with a directed oxygen-containing gas flow and burning, characterized in that the ground coal is heated to a semi-coking temperature of at least 500 ° C, volatile gaseous hydrocarbons are released from it, which further divided into liquid and gaseous fractions by condensation, and the semi-coke obtained by heating the ground coal is mixed with the directed oxygen-containing gas flow and burned.
2. The method according to claim 1, characterized in that the drying of the ground coal is carried out simultaneously with the grinding of the coal.
3. The method according to claim 1, characterized in that the milled coal is heated to a semi-coking temperature by mixing it with a gaseous heat carrier.
4. The method according to claim 1, characterized in that the ground coal is heated to the temperature of semi-coking by mixing it with a solid heat carrier having a temperature of 800-1300°C.
5. The method according to claim 3, characterized in that the gaseous heat carrier are gases formed during the combustion of at least a portion of volatile gaseous hydrocarbons.
6. The method according to claim 3, characterized in that the gaseous coolant is the gases formed during the combustion of at least part of the resulting semi-coke.
7. The method according to claim 4, characterized in that the solid heat carrier is the resulting semi-coke.
8. The method according to claim 4, characterized in that the solid heat carrier is quartz sand.
9. The method according to claim 4, characterized in that the solid heat carrier is a ceramic dispersed material.
10. The method according to claim 4, characterized in that the solid heat carrier is coal.
11. The method according to claim 4, characterized in that the solid heat carrier is an oxide of an inorganic substance with a fraction size of 0.5-5 mm.
12. The method according to claim 9, or 10, or 12, characterized in that the coolant after use is separated from the semi-coke by sieving.
13. The method according to claim 1, characterized in that the gaseous fraction of volatile hydrocarbons is completely or partially burned.
14. The method according to claim 13, characterized in that the gaseous fraction of volatile hydrocarbons is purified from sulfur-containing substances before combustion.
15. The method according to claim 1, characterized in that the heating of the ground coal to the temperature of semi-coking is carried out in a vortex chamber by mixing it with hot gas.
Answers to paragraph 19
1. What are the main natural sources of hydrocarbons you know? Oil, natural gas, shale, coal.
2. What is the composition of natural gas? Show on the geographical map the most important deposits: a) natural gas; b) oil; c) coal.
3. What advantages does natural gas have over other fuels? For what purposes is natural gas used in the chemical industry? Natural gas, compared to other sources of hydrocarbons, is the easiest to extract, transport and process. In the chemical industry, natural gas is used as a source of low molecular weight hydrocarbons.
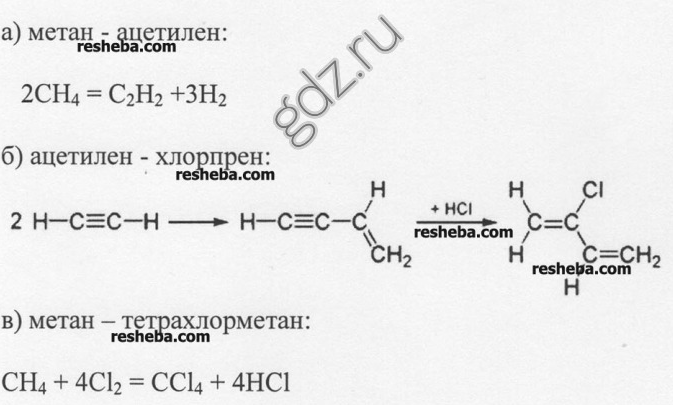
4. Write the equations for the reactions of obtaining: a) acetylene from methane; b) chloroprene rubber from acetylene; c) carbon tetrachloride from methane.
5. What is the difference between associated petroleum gases and natural gas? Associated gases are volatile hydrocarbons dissolved in oil. Their isolation occurs by distillation. Unlike natural gas, it can be released at any stage of the development of an oil field.
6.Describe the main products obtained from associated petroleum gases. Main products: methane, ethane, propane, n-butane, pentane, isobutane, isopentane, n-hexane, n-heptane, hexane and heptane isomers.
7. Name the most important oil products, indicate their composition and areas of their application.
8. What lubricating oils are used in production? Gear oils, industrial oils, cutting oils for machine tools, etc.
9. How is oil distillation carried out?
10. What is oil cracking? Make an equation for the reactions of hydrocarbon splitting and in this process.
11. Why is it possible to obtain no more than 20% of gasoline during direct distillation of oil? Because the content of the gasoline fraction in oil is limited.
12. What is the difference between thermal cracking and catalytic cracking? Give a description of the gasolines of thermal and catalytic cracking. In thermal cracking, it is necessary to heat the reactants to high temperatures, in catalytic cracking, the introduction of a catalyst reduces the activation energy of the reaction, which can significantly reduce the reaction temperature.
13. How can one practically distinguish cracked gasoline from straight run gasoline? Cracked gasoline has a higher octane number than straight run gasoline, i.e. more resistant to detonation and recommended for use in internal combustion engines.
14. What is aromatization of oil? Write reaction equations that explain this process.
15. What are the main products obtained by coking coal? Naphthalene, anthracene, phenanthrene, phenols and coal oils.
16. How is coke produced and where is it used? Coke is a gray porous solid product obtained by coco-coking coal at temperatures of 950-1100 without oxygen. It is used for iron smelting, as a smokeless fuel, an iron ore reducing agent, and a baking powder for charge materials.
17. What are the main products obtained: a) from coal tar; b) from tar water; c) from coke oven gas? Where are they applied? What organic substances can be obtained from coke oven gas? a) benzene, toluene, naphthalene - chemical industry b) ammonia, phenols, organic acids - chemical industry c) hydrogen, methane, ethylene - fuel.
18. Recall all the main ways to obtain aromatic hydrocarbons. What is the difference between the methods of obtaining aromatic hydrocarbons from the coking products of coal and oil? Write the equations of the corresponding reactions. They differ in the methods of obtaining: primary oil refining is based on the difference in the physical properties of various fractions, and coking is based purely on the chemical properties of coal.
19. Explain how, in the process of solving energy problems in the country, the ways of processing and using natural hydrocarbon resources will be improved. Search for new energy sources, optimization of oil production and refining processes, development of new catalysts to reduce the cost of all production, etc.
20. What are the prospects for obtaining liquid fuel from coal? In the future, obtaining liquid fuel from coal is possible, provided that the cost of its production is reduced.
Task 1. It is known that the gas contains 0.9 methane, 0.05 ethane, 0.03 propane, 0.02 nitrogen in volume fractions. What volume of air is required to burn 1 m3 of this gas under normal conditions?

Task 3. Calculate what volume (in l) and what mass (in kg) of carbon monoxide (IV) will be obtained by burning 5 moles of octane (n.o.).
2 Hydrogenation
Brown coal hydrogenation is a process of direct processing of coal into synthetic fuels of liquid and gaseous states of aggregation, which occurs at high pressure and relatively high temperature.
This direction of coal processing is being explored in different countries of the world.Abroad, this technology has received the greatest industrial introduction in South Africa, where four plants operate, with a total annual capacity of about 8-10 million tons of liquid fuel. The work is carried out using the patented SASOL technology based on the improved Fischer-Tropsch method. Given that SASOL has a policy of maintaining high payments for the right to use the technology, this leads to a high cost of its industrial implementation in other countries.[]
Preparation of brown coal includes crushing, drying, preparation of coal-hydrogenate paste. Grinding is carried out to a particle size of less than 0.1 mm - to increase the reactivity of the surface, it is implemented in disintegrators. In this case, the external specific surface increases by 20-30 times, the volume of transitional pores - by 5-10 times. The coal is then dried. The pores are filled with moisture, which prevents the penetration of reagents into the coal matter, it is released during the process in the reaction zone, reducing the partial pressure of H2, and also increases the amount of wastewater. For drying, tubular steam dryers, vortex chambers, dryer pipes are used in which coal is dried to a residual moisture content of 1.5%. The heat carrier is hot flue gases with a minimum O2 content (0.1-0.2%) so that the coal does not undergo oxidation. Coal is not heated above 150-200 ° C to avoid a decrease in reactivity.
Requirements for brown coal fed for liquefaction
On the basis of a large experimental material, it has been proved that coal with good hydraulicity contains from 65 to 85% C, more than 5% H, and has more than 30% volatile (V) yield. Rational moisture content of the initial coal for the hydrogenation process - Wrt = 10-15%, ash content Ad = 10-12%, value d
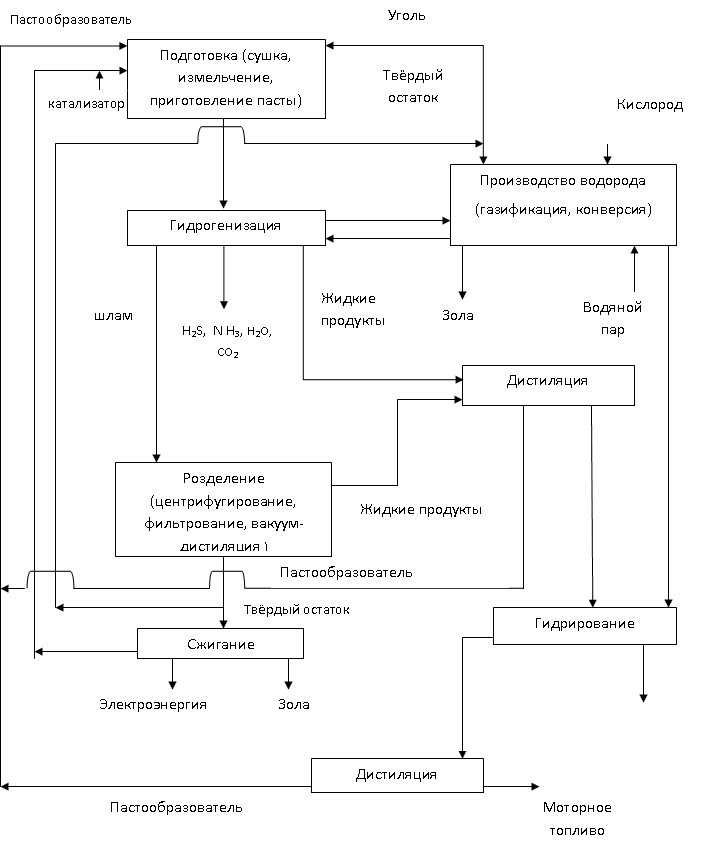
The most common hydrogenation scheme is shown in Figure 1.2 []
Rice. 1.2 - Scheme for obtaining synthetic liquid fuel from brown coal
Dynamics of gas consumption from coal in the world
| Intended use | Usage in 2001, MW for gas | Share in 2001, % | Commissioned before the end of 2004, MW for gas | Annual increase in capacity in 2002-2004, % |
| Chemical production | 18 000 | 45 | 5 000 | 9,3 |
| Intracycle gasification (electricity generation) | 12 000 | 30 | 11 200 | 31 |
| Fischer-Tropsch synthesis | 10 000 | 25 | ||
| TOTAL | 40 000 | 100 | 17 200 | 14,3 |
The given data clearly demonstrate the acceleration of the dynamics of the involvement of coal gasification in the global industry. The increased interest in intracycle gasification of coal in developed countries is due to two reasons.
Firstly, thermal power plants with intracycle gasification are environmentally less dangerous. Thanks to gas pre-treatment, emissions of sulfur oxides, nitrogen oxides and particulate matter are reduced.
Secondly, the use of a binary cycle can significantly increase the efficiency of the power plant and, consequently, reduce the specific fuel consumption.
In table. Table 2 shows the characteristic values of specific emissions and efficiency for TPPs with intracycle gasification and for TPPs with traditional coal combustion.
table 2
Specific Emissions and Efficiency for Thermal Power Plants with Intercycle Gasification and Conventional Coal Combustion
| Parameters | Traditional coal-fired power plant | TPP with intracycle gasification |
| The concentration of harmful substances in flue gases (for a coal-fired thermal power plant - according to the European standard), mg / m3 - SOx — NOx — Solid particles | 130 150 16 | 10 30 10 |
| Electrical efficiency, % | 33-35 | 42-46 |
It should be noted that the specific capital costs when using intracycle gasification are about 1500 US dollars per 1 kW with the prospect of reducing to 1000-1200 US dollars, while for a traditional coal-fired thermal power plant, the specific capital costs are about 800-900 US dollars per 1 kW. It is clear that a thermal power plant with intracycle gasification of solid fuel is more attractive in the presence of environmental restrictions at the location and when using rather expensive fuel, since the fuel consumption per 1 kW is reduced.
These conditions are typical for developed countries.At present, the use of intracycle gasification of solid fuels is considered the most promising direction in the energy sector.
3.3 Engineering developments over the past century
At present, the following most cost-effective areas of application of the gasification method have been identified:
— gasification of sulphurous and high-ash fuels with subsequent combustion of the resulting gases at powerful thermal power plants. The coals mined annually in Russia contain about 10 million tons of sulfur, most of which, when burned, is released into the atmosphere in the form of toxic sulfur oxides and carbon sulfide. During the gasification of sulfurous coals, hydrogen sulfide is formed, which can be relatively easily extracted and then processed into commercial sulfur or sulfuric acid.
— gasification of solid fuels for large-scale production of natural gas substitutes. This direction is of the greatest importance for local gas supply to areas remote from natural gas and oil fields or from main pipelines.
— gasification of solid fuels in order to obtain synthesis gas, reducing gases and hydrogen for the needs of the chemical, petrochemical and metallurgical industries.
The gasification process depends on many factors that affect the composition of the resulting gas and its calorific value. In this regard, there is still no single generally accepted classification of methods for implementing the process under consideration. Below is one of the possible classification options.
By type of blast (gasifying agent): air, air-oxygen, steam-air, steam-oxygen.
By pressure: at atmospheric pressure, at elevated pressure.
· by the size of fuel particles: gasification of coarse-grained (lump), fine-grained and pulverized fuel.
· according to the design features of the reaction zone: in a fixed dense layer of fuel, in a fluidized layer of fuel, in a pulverized coal flame.
by the method of removing ash: in solid form, in the form of liquid slag.
By the method of heat supply: with partial combustion of fuel in a gas generator, with mixing fuel with a preheated solid, liquid or gaseous heat carrier (regenerative heating), with heat supply through the wall of the apparatus (recuperative heating).
Carbon monoxide, metal carbonyls and the 18 electron rule
Numerous
syntheses based on carbon monoxide and
hydrogen represent a huge
practical as well as theoretical
interest, as allow from two
the simplest substances to receive the most valuable
organic compounds. And here
catalysis plays an important role
transition metals that are capable of
activate inert CO molecules and
H2.
Activation of molecules is their translation into
more reactive state.
It should be noted in particular that in the transformations
synthesis gas has been widely developed
a new type of catalysis - catalysis by complexes
transition metals or metal complex
catalysis (see the article by O.N. Temkin
).
So
Is the CO molecule inert? Representation
about the inertness of carbon monoxide
conditional character. Back in 1890 Mond
obtained from metallic nickel and
carbon monoxide first carbonyl
metal compound, volatile liquid
with a boiling point of 43 ° C - Ni (CO)4 .
The history of this discovery is interesting.
which can be classified as random. Mond,
investigating the causes of rapid corrosion
nickel reactors in production
soda from NaCl, ammonia and CO2,
found that the cause of corrosion is
presence in CO2 impurities
carbon monoxide, which reacted
with nickel to form tetracarbonyl
Ni(CO)4 .
This discovery allowed Mond to further
develop methods for purifying nickel
through the production of a volatile carbonyl
nickel and its subsequent thermal
decomposition again to nickel and CO. Across
25 years also accidentally discovered carbonyl
iron - Fe(CO)5.
When BASF opened a long-forgotten
steel cylinder with CO, found at the bottom
yellow liquid - iron pentacarbonyl,
which gradually developed into
the result of a metal reaction
iron with CO under high pressure.
Because metal carbonyls are
highly toxic compounds, initially
the attitude of chemists towards them was very
cool, but in the future were
amazing properties discovered, including
including catalytic, which determined
their wide application, especially in chemistry
carbon monoxide. Note that many
metals in a finely dispersed state
can react directly
with carbon monoxide, but in this way
receive only nickel carbonyls and
gland. Carbonyls of other metals
obtained by restoring their compounds
in the presence of CO at high
pressures.
Compound
transitional carbonyl complexes
metals can be predicted based on
18 electron rule, according to which
the complex will be stable if the sum
valence electrons of metal and electrons,
provided by the ligand, in our case
CO, will be equal to 18, since in this case
electronic configuration corresponds
stable configuration of atoms
noble gases (krypton).
Molecule
carbon monoxide has lone
pairs of electrons, while a pair of electrons
on carbon can be provided
to form a bond with the metal
donor-acceptor type. As
For example, consider the structure of carbonyls
iron and nickel Fe(CO)5 and
Ni(CO)4.
The iron and nickel atoms have, respectively,
8 and 10 valence electrons, and to fill
electron shell of an atom before configuration
noble gas atom krypton
10 and 8 electrons are missing, and therefore
in the formation of carbonyls to the iron atom
must provide electron pairs
five CO molecules, and a nickel atom
four.
transitional
metals that have an odd number of valences
electrons, form binuclear
carbonyl complexes. So, for cobalt,
having nine valence electrons
to stable electronic configuration
missing nine electrons. single core
complexes by taking four pairs
from CO molecules will have unpaired
electrons, and such particles of the radical
characters interact with each other.
to form a metal-metal bond, and
resulting in the formation of a dimer
Co complex2(CO)8.
Interaction
or coordination of carbon monoxide with
metal leads to a redistribution
electron density not only on CO,
but also on metal, which significantly affects
on the reactivity of the carbonyl
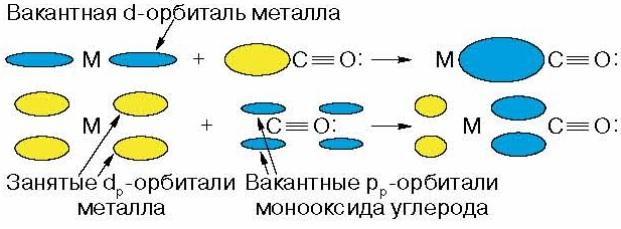
complex. The most common is
called linear type of coordination
CO:
At
this is not only the s-interaction
due to a free pair of electrons
carbon, but also p-interaction due to
electron transfer from the d-orbital of the metal
to energetically available vacant
carbon orbitals:
Relevance
Thus, there is a need to develop such a technology for the primary processing and agglomeration of brown coal, which would take into account the specific properties of the original brown coal, the conditions of the process of hydraulic transportation of coal and the requirements for the characteristics of coal raw materials in further operations for its processing - pyrolysis, combustion, liquefaction, briquetting, dehydration. The solution to this problem can be the technology of treating coal with oil reagents - oil aggregation.
Selective oil aggregation of coal combines a set of processes for structuring a thin polydisperse coal phase in an aqueous medium using oil reagents.The processes of selective oil aggregation of coal are based on the mechanism of adhesive interaction between an oleophilic coal surface and oils, which results in its selective wetting and aggregation in a turbulent water flow. Hydrophilic particles that are not wetted by oil are not included in the structure of the aggregates, which allows them to be isolated in the form of a rock suspension.
The treatment of brown coal by selective oil aggregation eliminates its disintegration and soaking, "preserving" organic matter in hydrophobic aggregates, which are easily dehydrated by mechanical methods and are a good raw material for pyrolysis, briquetting, and gasification.
1 Briquetting
Briquetting of coal is a physical and chemical process of obtaining a mechanically and thermally strong high-quality product - a briquette having a given geometric shape, size and weight.
The technological process of briquetting brown coal without a binder consists of the following operations: preparation of coal in terms of size and moisture, and pressing.
Technological indicators that lignite briquettes must comply with: briquette weight 100-500 g, mechanical abrasion strength 75-80%, compression and bending 70-90 and 10-15 MPa, respectively, moisture absorption 3-4%, calorific value 24000-30000 kJ / kg, ash content 10-25%.[]