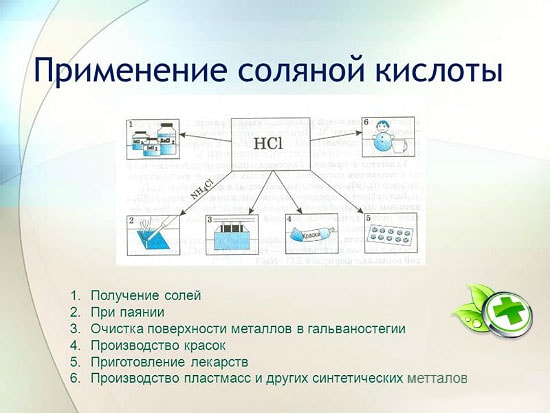
The use of hydrochloric acid
Hydrochloric acid is widely used in industry for extracting metals from ores, pickling metals, etc. It is also used in the manufacture of soldering liquid, in the deposition of silver, and as an integral part of aqua regia.
The scale of the use of hydrochloric acid in industry is less than that of nitric acid. This is due to the fact that hydrochloric acid causes corrosion of steel equipment. In addition, its volatile vapors are quite harmful and also cause corrosion of metal products. This must be taken into account when storing hydrochloric acid. Hydrochloric acid is stored and transported in rubber-lined tanks and barrels, i.e. in vessels, the inner surface of which is covered with acid-resistant rubber, as well as in glass bottles and polyethylene utensils.
Hydrochloric acid is used to produce chlorides of zinc, manganese, iron and other metals, as well as ammonium chloride. Hydrochloric acid is used to clean the surfaces of metals, vessels, wells from carbonates, oxides and other sediments and contaminants. In this case, special additives are used - inhibitors that protect the metal from dissolution and corrosion, but do not delay the dissolution of oxides, carbonates and other similar compounds.
HCl is used in the industrial production of synthetic resins, rubbers. It is used as a raw material in the production of methyl chloride from methyl alcohol, ethyl chloride from ethylene, and vinyl chloride from acetylene.
First aid and treatment methods
If signs of poisoning are found, an ambulance must be called. At home, it is allowed to carry out activities aimed at improving the condition of the victim. First aid for hydrochloric acid poisoning should be carried out quickly to reduce the risk of negative consequences.
Events:
- If the salt compound gets on the skin, the damaged areas are washed with plenty of cool water. The processing time is at least half an hour.
- In case of intoxication with vapors, the victim is provided with access to fresh air, windows are opened, tight clothes are unbuttoned.
- It is recommended to monitor the patient's condition, in the absence of signs of life, resuscitation is carried out.
- The victim from the vapors is allowed to drink warm tea, water. It is recommended to rinse the nose and mouth with cool water.
- In case of an overdose resulting from ingestion of acid, an ice pack is placed on the abdomen to prevent or reduce possible bleeding.
- No medicinal products are allowed. It is allowed to give the patient a glass of water (you can mineral alkaline). Drinking liquid is required in small sips.
- It is not allowed to wash the stomach, try to induce vomiting at home. Such first aid can lead to the development of burns of the throat, bleeding.
Treatment is carried out in a medical institution under the supervision of specialists.
Therapy:
- Cleansing the stomach with a probe,
- Use of droppers with medicinal solutions,
- Prescribing medications to relieve pain
- The use of medicines aimed at restoring the functioning of organs and systems,
- If necessary, oxygen inhalation and artificial ventilation of the lungs,
- Carrying out resuscitation therapy in the absence of signs of life,
- Selection of vitamins and special nutrition.
Treatment is carried out in intensive care, and then in a hospital. The duration depends on the patient's condition and the degree of poisoning.
Burns and poisoning
As effective as this remedy is, it is dangerous. Hydrochloric acid, depending on the concentration, can provoke chemical burns of four degrees:
- There is only redness and pain.
- There are bubbles with a clear liquid and swelling.
- Formed necrosis of the upper layers of the skin.Blisters fill with blood or cloudy contents.
- The lesion reaches the tendons and muscles.
If the substance somehow got into the eyes, it is necessary to rinse them with water, and then with a soda solution. But in any case, the first thing to do is to call an ambulance.
The ingestion of acid inside is fraught with acute pains in the chest and abdomen, swelling of the larynx, vomiting bloody masses. As a result, severe pathologies of the liver and kidneys.
And the first signs of poisoning in pairs include a dry frequent cough, choking, damage to the teeth, burning in the mucous membranes and abdominal pain. The first emergency aid is washing and rinsing the mouth with water, as well as access to fresh air. Only a toxicologist can provide real help.
Properties of the chemical spectrum
Acid interacts with many metals, salts. It is considered quite strong and is on a par with chamois. The main reaction manifests itself in all groups of metals located to the left of hydrogen (magnesium, iron, zinc - electrical potentials).
Hydrogen chloride solution in dilute form reacts with salts, but only with those formed by less strong acids. Known to all sodium and calcium carbonate, after interaction with it, they decompose into water and carbon monoxide.
Nitric acid
- a qualitative reaction to saline solution. To obtain it, it is necessary to add silver nitrate to this reagent, as a result, a white precipitate will form, from which a nitrogen substance is obtained.
With the help of this mixture of water and hydrogen, many interesting experiments are carried out. For example, dilute it with ammonia. As a result, you will get white smoke, thick, having the consistency of small crystals. Methylamine, aniline, manganese dioxide, potassium carbonate are reagents that are also affected by acid.
How and why is it used
Perhaps, this is rightfully one of the important substances that is found and necessary in almost all branches of human life.
Application area localization:
- Metallurgy. Surface cleaning from oxidized areas, rust dissolution, pre-soldering treatment, tinning. Hydrochloric acid helps to extract small inclusions of metals from ores. Zirconium and titanium are obtained using the method of converting oxides into chlorides.
- Food technology industry. A low concentration solution is used as a food additive. Gelatin, fructose for diabetics contain a pure emulsifier. Ordinary soda also has a high content of this substance. On the packaging of goods you will see it under the name E507.
- The field of medicine. With an insufficient indicator of the acidic environment in the stomach and problems with the intestines. Low Ph leads to cancer. Even with proper nutrition, vitamins in abundance, the danger does not disappear, it is necessary to carry out tests to obtain juice from the gastric tract, because in an insufficiently acidic environment, useful substances are practically not absorbed, digestion is disturbed.
- Salt solution is used as an inhibitor - protection against dirt and infections, antiseptic action. For the manufacture of adhesive mixtures, ceramic products. It flushes heat exchangers.
- The procedure for purifying drinking water is also not complete without the participation of chlorine.
- Production of rubber, bleaching of fabric bases.
- You can care for your lenses with this solution.
- Mouthwash at home
- The substance is an excellent conductor of electricity.
How poisoning occurs
Hydrochloric acid is a colorless liquid with a characteristic pungent odor. One of the strongest acids, capable of dissolving some metals. Easily turns into a gas.
Hydrogen chloride is used in the textile industry, leather business, metallurgy of precious metals, in the production of glue, acids.
The substance is present in the stomach in minimal concentration. Acid contributes to the normalization of the digestive process, protects the body from harmful bacteria and microorganisms.
At a concentration exceeding 24%, hydrochloric acid can cause irreversible harm to the human body. Vapors formed upon contact with air cause irritation to the organs of the visual and respiratory systems.
There are several factors that can provoke the development of poisoning.
Factors:
Vapor intoxication is possible when working in rooms with poor ventilation,
Ingestion by negligence, more common in children,
The ingress of hydrochloric acid on the epidermis, mucous membranes in case of non-compliance with the rules for using the reagent.
Poisoning by a substance at home in adults occurs as a result of the use without protective equipment of the skin, eyes, and organs of the respiratory system. Intoxication can occur when acid is inaccurately transferred from one container to another.
Pharmacodynamics and pharmacokinetics
What is gastric acidity? This is a characteristic of the concentration of hydrochloric acid in the stomach. Acidity is expressed in pH
. Normally, acid should be produced in the composition of gastric juice and take an active part in the processes of digestion. Formula of hydrochloric acid: HCl
. It is produced by parietal cells located in the fundic glands, with the participation of H+/K+-ATPase
. These cells line the fundus and body of the stomach. The acidity of gastric juice itself is variable and depends on the number of parietal cells and the intensity of the processes of neutralization of the substance by the alkaline components of gastric juice. Concentration produced to - you are stable and equal to 160 mmol/l. A healthy person should normally produce no more than 7 and at least 5 mmol of a substance per hour.
With insufficient or excessive production of Hydrochloric Acid, diseases of the digestive tract occur, the ability to absorb certain microelements, such as iron, deteriorates. The drug stimulates the secretion of gastric juice, reduces pH
. Activates pepsinogen
, converts it into an active enzyme pepsin
. The substance has a beneficial effect on the acid reflex of the stomach, slows down the transition of incompletely digested food into the intestines. The processes of fermentation of the contents of the digestive tract slow down, pain and belching disappear, iron is better absorbed.
After oral administration, the drug is partially metabolized by saliva and gastric mucus, the contents of the duodenum 12. The unbound substance penetrates into the duodenum, where it is completely neutralized by its alkaline contents.
Another use of acid in everyday life
With an acidic composition, you can easily clean limescale and rust from faience plumbing, remove urinary stone and other contaminants. For a greater effect, an inhibitor (for example, urotropin) is added to the agent, which slows down the chemical reaction.
The procedure is carried out as follows: the acid is diluted with water until a 5% concentration is reached and an inhibitor is added at the rate of 0.5 g per 1 liter of liquid. The surface is treated with the resulting composition and left for 30-40 minutes (depending on the degree of contamination), after which it is washed with water.
A mild acid solution is also used to remove berry stains, ink or rust stains from fabrics. To do this, the material is soaked in the composition for a while, after which it is thoroughly rinsed and washed in the usual way.
Getting rid of scale in the kettle
For this purpose, a 3-5% hydrochloric acid solution is used, which is poured into a kettle and heated to 60-80°
C for 1-2 hours or until scale deposits disintegrate. After that, the scale becomes loose and is easily removed with a wooden spatula.
The effectiveness of the method is due to the fact that the reagent reacts with magnesium and calcium carbonates and converts them into soluble salts. The carbon dioxide released at the same time destroys the scale layer and makes it loose. After removing salt deposits, the dishes are thoroughly washed with clean water.
Important point!
This method is not suitable for descaling chipped and cracked enamel or aluminum kettles: this will corrode the metal and cause severe damage.
Getting a substance
Now we can talk about what they do to form hydrochloric acid.
First, by burning hydrogen in chlorine, the main component, gaseous hydrogen chloride, is obtained. which is then dissolved in water. The result of this simple reaction is the formation of a synthetic acid.
This substance can also be obtained from off-gases. These are chemical waste (side) gases. They are formed by a variety of processes. For example, when chlorinating hydrocarbons. The hydrogen chloride in their composition is called off-gas. And the acid thus obtained, respectively.
It should be noted that in recent years the share of the off-gas substance in the total volume of its production has been increasing. And the acid formed as a result of burning hydrogen in chlorine is displaced. However, in fairness, it should be noted that it contains fewer impurities.
Production use
It has a wide application in the metallurgical, food and medical industries.
- Metallurgy. Use in soldering, tinning and cleaning metals.
- Food industry. Application in the production of food acidity regulators, for example, E507.
- Electrotype. Used for pickling.
- Medicine. It finds its application in the production of artificial gastric juice.
Included in synthetic dyes. It is used in the production of cleaning and detergent products. But in liquids intended for domestic use, the concentration of sulfuric acid is negligible.
How hydrochloric acid is produced in the laboratory
The production of the substance is large-scale, the sale is free. In the conditions of laboratory experiments, a solution is produced by the action of high concentration sulfuric acid on ordinary kitchen salt (sodium chloride).
There are 2 methods for dissolving hydrogen chloride in water:
- Hydrogen is burned in chlorine (synthetic).
- Associated (off-gas). Its essence is in carrying out organic chlorination, dehydrochlorination.
The substance lends itself well to synthesis during the pyrolysis of waste from organochlorine. This happens as a result of the breakdown of hydrocarbons with a complete lack of oxygen. You can also use metal chlorides, which are the raw materials of inorganic substances. If there is no concentrated sulfuric acid (electrolyte), take diluted.
As for the extraction of the reagent in natural conditions, most often this chemical mixture can be found in the waters of volcanic waste. Hydrogen chloride is a component of the minerals sylvin (potassium chloride, looks like bones for games), bischofite. All these are methods to extract the substance in industry.
In humans, this enzyme is found in the stomach. A solution can be either an acid or a base. One of the most common methods of extraction is called sulfate.
Chemical properties
Hydrochloric acid, hydrogen chloride or hydrochloric acid - solution HCl
in water. According to Wikipedia, the substance belongs to the group of inorganic strong monobasic to-t. The full name of the compound in Latin: hydrochloric acid.
Formula of hydrochloric acid in chemistry: HCl
. In a molecule, hydrogen atoms combine with halogen atoms - Cl
. If we consider the electronic configuration of these molecules, it can be noted that compounds take part in the formation of molecular orbitals 1s
-hydrogen orbitals and both 3s
and 3p
-orbitals of an atom Cl
. In the chemical formula of hydrochloric acid 1s-
, 3s-
and 3r
-atomic orbitals overlap and form 1, 2, 3 orbitals. Wherein 3s
-orbital is not binding. There is a shift of the electron density to the atom Cl
and the polarity of the molecule decreases, but the binding energy of molecular orbitals increases (if we consider it along with other hydrogen halides
).
Physical properties of hydrogen chloride. It is a clear, colorless liquid that smokes when exposed to air. Molar mass of a chemical compound = 36.6 grams per mole. Under standard conditions, at an air temperature of 20 degrees Celsius, the maximum concentration of a substance is 38% by weight. The density of concentrated hydrochloric acid in this kind of solution is 1.19 g/cm³. In general, physical properties and characteristics such as density, molarity, viscosity, heat capacity, boiling point and pH
, strongly depend on the concentration of the solution. These values are discussed in more detail in the table of densities. For example, the density of Hydrochloric Acid 10% = 1.048 kg per liter. When solidified, the substance forms crystalline hydrates
different compositions.
Chemical properties of hydrochloric acid. What does hydrochloric acid react with? The substance interacts with metals that stand in front of hydrogen in a series of electrochemical potentials (iron, magnesium, zinc, and others). In this case, salts are formed and gaseous H
. Lead, copper, gold, silver and other metals to the right of hydrogen do not react with hydrochloric acid. The substance reacts with metal oxides producing water and a soluble salt. Sodium hydroxide under the action of to-you forms and water. The neutralization reaction is characteristic of this compound.
Dilute Hydrochloric Acid reacts with metal salts, which are formed by weaker acids. For instance, propionic acid
weaker than salt. The substance does not react with stronger acids. and sodium carbonate
will form after reaction with HCl
chloride, carbon monoxide and water.
For a chemical compound, reactions with strong oxidizing agents are characteristic, with manganese dioxide
, potassium permanganate
: 2KMnO4 + 16HCl = 5Cl2 + 2MnCl2 + 2KCl + 8H2O
. The substance reacts with ammonia
, which produces thick white smoke, which consists of very small crystals of ammonium chloride. The mineral pyrolusite also reacts with hydrochloric acid, as it contains manganese dioxide
: MnO2+4HCl=Cl2+MnO2+2H2O
(oxidation reaction).
There is a qualitative reaction to hydrochloric acid and its salts. When a substance interacts with silver nitrate
a white precipitate silver chloride
and formed nitric acid
. Interaction reaction equation methylamine
with hydrogen chloride looks like this: HCl + CH3NH2 = (CH3NH3)Cl
.
A substance reacts with a weak base aniline
. After dissolving aniline in water, hydrochloric acid is added to the mixture. As a result, the base dissolves and forms aniline hydrochloride
(phenylammonium chloride
): (С6Н5NH3)Cl
. The reaction of interaction of aluminum carbide with hydrochloric acid: Al4C3+12HCL=3CH4+4AlCl3
. Reaction equation potassium carbonate
with which it looks like this: K2CO3 + 2HCl = 2KCl + H2O + CO2.
Application
in metallurgy for the extraction of ores, removal of rust, scale, dirt and oxides, soldering and tinning;
in the manufacture of synthetic rubbers and resins;
in electroplating;
as an acidity regulator in the food industry;
to obtain metal chlorides;
to obtain chlorine;
in medicine for the treatment of insufficient acidity of gastric juice;
as a cleaner and disinfectant.
- (HCl), an aqueous solution of hydrogen chloride, a colorless gas with a pungent odor. Obtained by the action of sulfuric acid on common salt, as a by-product of the chlorination of hydrocarbons, or by the reaction of hydrogen and chlorine. Hydrochloric acid is used for ... ... Scientific and technical encyclopedic dictionary
Hydrochloric acid
- - HCl (SC) (hydrochloric acid, hydrochloric acid, hydrogen chloride) is a solution of hydrogen chloride (HCl) in water, an antifreeze additive. It is a colorless liquid with a pungent odor, without suspended particles. ... ... Encyclopedia of terms, definitions and explanations of building materials
HYDROCHLORIC ACID
- (hydrochloric acid) solution of hydrogen chloride in water; strong acid. A colorless liquid fuming in air (technical hydrochloric acid is yellowish due to impurities of Fe, Cl2, etc.). The maximum concentration (at 20 ° C) is 38% by mass, ... ... Big Encyclopedic Dictionary
HYDROCHLORIC ACID
- (Acidum muriaticum, Acid, hydrochloricum), a solution of hydrogen chloride (HC1) in water. In nature, it occurs in the water of certain sources of volcanic origin, and is also found in gastric juice (up to 0.5%). Hydrogen chloride can be obtained ... Big Medical Encyclopedia
HYDROCHLORIC ACID
- (hydrochloric acid, hydrochloric acid) strong monobasic volatile acid with a pungent odor, an aqueous solution of hydrogen chloride; the maximum concentration is 38% by mass, the density of such a solution is 1.19 g/cm3. Used in ... ... Russian encyclopedia of labor protection
HYDROCHLORIC ACID
- (hydrochloric acid) HCl aqueous solution of hydrogen chloride, strong monobasic acid, volatile, with a pungent odor; impurities of iron, chlorine stain it yellowish. The concentrated S. to sell contains 37% ... ... Big Polytechnic Encyclopedia
hydrochloric acid
— noun, number of synonyms: 1 acid (171) ASIS Synonym Dictionary. V.N. Trishin. 2013 ... Synonym dictionary
HYDROCHLORIC ACID
Modern Encyclopedia
Hydrochloric acid
- HYDROGENIC ACID, aqueous solution of hydrogen chloride HCl; a fuming liquid with a pungent odor. Hydrochloric acid is used to obtain various chlorides, pickling metals, processing ores, in the production of chlorine, soda, rubbers, etc. ... ... Illustrated Encyclopedic Dictionary
hydrochloric acid
- (hydrochloric acid), a solution of hydrogen chloride in water; strong acid. A colorless liquid "fuming" in air (technical hydrochloric acid is yellowish due to impurities of Fe, Cl2, etc.). Maximum concentration (at 20 ° C) 38% by mass, ... ... Encyclopedic Dictionary
What is the danger of intoxication
Hydrochloric acid is a particular danger to the human body. In case of poisoning with such a substance, serious complications and violations of the functionality of the body may develop.
Complications:
- Violation of the liver, as a result of toxic hepatitis,
- Bleeding in the stomach due to the destroyed walls of the organ,
- Shock from pain when acid enters a large area,
- In case of contact with the eyes, visual impairment,
- Serious malfunction of the kidneys,
- Respiratory failure, suffocation, shortness of breath,
- The development of a coma.
Similar consequences develop gradually depending on the degree of poisoning.
Conclusion
If you follow the precautions and safety rules, hydrochloric acid will become an indispensable assistant in everyday life. And you can buy it at the most affordable prices in our company.
Like acids. The educational program provides for memorization by students of the names and formulas of six representatives of this group. And, looking through the table provided by the textbook, you notice in the list of acids the one that comes first and interested you in the first place - hydrochloric acid. Alas, in the classroom at school, neither the property nor any other information about it is studied. Therefore, those who are eager to gain knowledge outside the school curriculum are looking for additional information in all sorts of sources. But often, many do not find the information they need. And so the topic of today's article is dedicated to this particular acid.
Definition
Hydrochloric acid is a strong monobasic acid. In some sources, it may be called hydrochloric and hydrochloric, as well as hydrogen chloride.
Physical properties
It is a colorless and fuming caustic liquid in the air (photo on the right). However, technical acid has a yellowish color due to the presence of iron, chlorine and other additives in it. Its largest concentration at a temperature of 20 ° C is 38%. The density of hydrochloric acid with such parameters is 1.19 g/cm 3 . But this compound in varying degrees of saturation has completely different data.With a decrease in concentration, the numerical value of molarity, viscosity and melting point decrease, but the specific heat capacity and boiling point increase. Solidification of hydrochloric acid of any concentration gives various crystalline hydrates.
Chemical properties
All metals that come before hydrogen in the electrochemical series of their voltage can interact with this compound, forming salts and releasing hydrogen gas. If they are replaced by metal oxides, then the reaction products will be soluble salt and water. The same effect will be in the interaction of hydrochloric acid with hydroxides. If, however, any salt of metals (for example, sodium carbonate) is added to it, the residue of which was taken from a weaker acid (carbonic), then chloride of this metal (sodium), water and gas corresponding to the acid residue (in this case, carbon dioxide) are formed. .
Receipt
The compound now discussed is formed when hydrogen chloride gas, which can be obtained by burning hydrogen in chlorine, is dissolved in water. Hydrochloric acid, which was obtained using this method, is called synthetic. Off-gases can also serve as a source for obtaining this substance. And such hydrochloric acid will be called off-gas. Recently, the level of production of hydrochloric acid using this method is much higher than its production by a synthetic method, although the latter gives the compound in a purer form. These are all ways of getting it in industry. However, in laboratories, hydrochloric acid is produced in three ways (the first two differ only in temperature and reaction products) using various types of chemical interactions, such as:
- Effect of saturated sulfuric acid on sodium chloride at a temperature of 150 o C.
- The interaction of the above substances under conditions with a temperature of 550 ° C and above.
- Hydrolysis of aluminum or magnesium chlorides.
Application
Hydrometallurgy and electroforming cannot do without the use of hydrochloric acid, where it is needed, to clean the surface of metals during tinning and soldering and to obtain chlorides of manganese, iron, zinc and other metals. In the food industry, this compound is known as food additive E507 - there it is an acidity regulator necessary in order to make seltzer (soda) water. Concentrated hydrochloric acid is also found in the gastric juice of any person and helps to digest food. During this process, its degree of saturation decreases, because. this composition is diluted with food. However, with prolonged fasting, the concentration of hydrochloric acid in the stomach gradually increases. And since this compound is very caustic, it can lead to stomach ulcers.
Conclusion
Hydrochloric acid can be both beneficial and harmful to humans. Its contact with the skin leads to severe chemical burns, and the vapors of this compound irritate the respiratory tract and eyes.
But if you handle this substance carefully, it can come in handy more than once in